Characterization and Assessment of KCNU1/SLO3 as a Potential Non-hormonal Contraceptive Target
Introduction
Project Proposal
The Structural Genomics Consortium (SGC) has recently embarked on its first research project in reproductive biology as a part of the SGC’s new open science Women’s and Children’s Health Program (WCHP). This program, which was generously funded by the Bill & Melinda Gates Foundation will focus on drug discovery for childhood diseases and development as well as reproductive biology and disease. One of the first initiatives of WCHP is to identify and characterize protein targets for non-hormonal contraceptive agents.
Throughout this article we will use the term people with uteruses (PWU) to encapsulate women, transgender men, and non-binary persons, all of whom experience unintended pregnancies. We will use female reproductive system to refer to the system, consisting of a uterus and ovaries, which can cause PWU to become pregnant. We note that the biological reproductive system may have no bearing on a person’s gender.
Contraception as Basic and Essential Healthcare
Access to contraception supports PWU’s autonomy over when and if to have children, which in turn has allowed PWU to pursue a career of their choosing, as well as financial stability. In 2005, Hock published research indicating that unrestricted access to contraceptives led to increased enrollment and graduation of PWU in college1. The ability to plan, delay, and space out births seems to empower PWU to achieve their educational and career goals2. Furthermore, delaying births can reduce the pay gap that exists between working PWUs and their peers who do not have children, as well as the gender pay gap2. Some disadvantages of unplanned births include: increased conflict and dissatisfaction in relationships, increased likelihood of experiencing anxiety and depression, obstacles to obtaining a secondary education, reduced earnings, and less preparation and financial resources to support the child2.
Birth control and contraceptive use not only supports PWU who want to avoid pregnancy; in many cases, birth control is prescribed as a method to treat another condition. Some examples include: to reduce pain in those with endometriosis, to protect against sexually transmitted infections such as pelvic inflammatory disease, for polycystic ovary syndrome, and to reduce hormonal acne. Birth control also impacts our society, economy, and planet. PWU who experience an unintended pregnancy are at a significant risk of mortality, morbidity, and financial hardship3. Additionally, our global population is increasing concurrent with an increase in average life expectancy, draining on our planet’s resources and stressing economies that attempt to support more people, for longer.
The Need for Effective Non-hormonal Contraceptives
Birth control enhances the lives of PWU and their partners, and advances our societies and economies; thus, it is imperative that PWUs have equal and uncomplicated access to birth control. As it stands, unintended pregnancy is a critical global issue, yet the rates of unintended pregnancy are higher among lower-income PWU and minorities3. Although access to contraception is an important factor to consider, other factors include the use of less efficient contraceptive methods, lack of patient education and understanding, as well as health concerns3. For example, according to a study published by the World Health Organization in 2020, approximately 40% of PWU using short-acting or long-acting modern contraceptive methods discontinued use due to side effects and health concerns4.
Currently, the most successful forms of birth control are permanent surgical procedures and hormonal methods. Unfortunately, these more effective birth control methods come at a price: extensive side effects. As most hormonal birth control methods target the female reproductive system, PWU are disproportionately affected by the negative side effects which range from moderate, such as dizziness, weight gain, painful cramping, diminished libido, and mood swings, to severe or even deadly, such as blood clots, high blood pressure, and depression. Due to the extensive side effects of hormonal birth control, it should be of the upmost priority to develop safer and more effective non-permanent contraceptive methods that align with PWU’s needs and preferences. Moreover, it is critical that researchers expand their focus to non-hormonal contraceptives within the male reproductive system.
Our contribution to the identification of non-hormonal contraceptive targets
The objective of this research project is to identify potential targets for non-hormonal contraceptives. This is no insignificant task: there are many steps on the path to such a discovery, and the process takes over a decade. Our efforts will be focused on the initial research, for instance, identifying potential proteins of interest and characterizing their structure and function, which can then be used as a guide to design small molecule inhibitors capable of disrupting protein functions. Another area of our research will be to create chemical probes: selective and cell-permeable inhibitors of protein function. Chemical probes are important to the early stages of drug discovery, as they allow preclinical target validation to be performed5. We will also work to create target-enabling packages (TEPs) to validate our protein targets for the future development of non-hormonal contraceptives. TEPs provide knowledge on protein targets such as production methods, biochemical assays for activity, structures, etc., to allow rapid biochemical and chemical characterization of proteins that are linked to diseases6. As we discover chemical probes and assays for our target protein, we will publish our results to open access forums, providing researchers with a starting point for drug discovery.
KCNU1: A potential contraceptive target
Although most contraceptive strategies have focused on the female reproductive system, there is great interest in developing contraceptives for the male reproductive system. Ion channels in sperm are of interest as viable targets for non-hormonal contraceptives for several reasons7. First, they are promising drug targets due to their involvement in various pathophysiologies as well as their roles in sperm capacitation and the acrosomal reaction7,8. Second, they contain druggable sites at cell surfaces, and third, proteins that respond to ion signaling are essential to sperm cells which must adapt to changing environments and respond to cues from the female reproductive tract7. It is worth noting that approximately 15% of current drug targets are ion channels7.
One potential target is potassium/calcium-activated channel subfamily U member I (KCNU1), sometimes referred to as SLO3, a potassium channel that is expressed solely in mammalian sperm8,9. This channel is essential to reproduction, as it has been observed in a knockout mouse model that the absence of KCNU1 leads to infertility8,9. KCNU1 is a homolog of SLO1, a Ca2+-activated channel, and sequence analysis suggests that KCNU1, which only exists in mammals, resulted from a gene duplication of Slo19. KCNU1 is distinct from SLO1 in that it is not activated by binding of intracellular Ca2+, but instead responds to increases in intracellular pH and membrane voltage8,9.
In addition to its individual functions, KCNU1 works in tandem with other proteins. For example, one study revealed that coexpression of a transmembrane protein member of the leucine-rich repeat containing family, LLRC52, with KCNU1 in Xenopus oocytes led to increased current, reduced activation rate for current, and a shift of KCNU1’s pH dependence to allow channel opening at lower pH values9. Also worth noting is that KCNU1-dependent hyperpolarization is necessary for Ca2+ influx through CATSPER, a sperm-specific Ca2+ channel10.
Knowledge of the crystal structure of KCNU1 will be of great use to researchers who want to identify druggable sites and perform docking experiments. KCNU1 consists of an N-terminal transmembrane voltage sensor and a large, C-terminal cytoplasmic structure that performs regulation by intracellular signals9. In 2012, Leonetti et al. published a crystal structure of the tetrameric gating ring formed by assembly of four of the cytoplasmic regions9. Within this ring, each of the four subunits consists of tandem regulator of K+ conductance (RCK) domains which interact through a flexible interface (Figure 1)9.
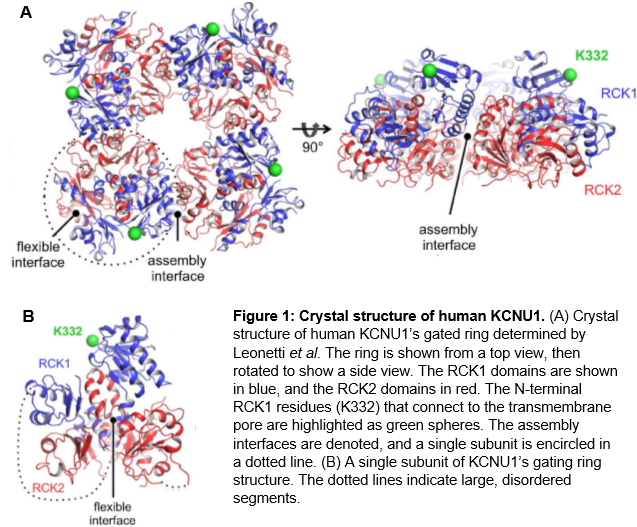 |
Methods
Protein Production
To design KCNU1 constructs for expression and purification, we used the AlphaFold predicted structure of full-length KCNU1 as well as the available crystal structure of KCNU1’s cytoplasmic domain. The predicted structure revealed an N-terminal transmembrane region (aa M1-K329 approx.) and a C-terminal cytoplasmic region (aa G330-L1149). Within the C-termini of the protein is a disordered region (aa K1061-L1149) as well as two large, disordered loops (Y602-H715 & L827-C856).
Informed by AlphaFold2 and the crystal structure, we designed domain boundaries for protein expression constructs. Transmembrane regions are very hydrophobic, allowing such regions to be stable within membranes, however, this makes recombinant expression challenging. The recombinant expression of membrane proteins often leads to misfolding and protein aggregation due to the hydrophobic regions of the protein. Therefore, we designed our constructs to exclude the transmembrane region of the protein, which appears to end around amino acid 330 (Figure 2). Disordered regions of proteins are likewise challenging to express, as disordered regions are often prone to aggregation, particularly at higher concentrations. As such, our protein constructs end at amino acid 1062.
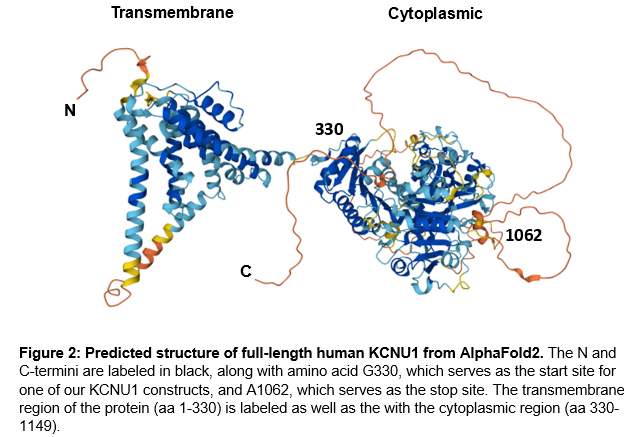
For our initial screen, we designed 7 protein constructs that started at G330 and ended at A1062 for recombinant expression in the baculovirus/SF9 insect cell system. Expression constructs were first tested on a small scale to determine which constructs generate soluble protein. Our initial test appeared to indicate successful protein expression for six constructs, so we proceeded to attempt to purify KCNU1 G330-A1062 with an N-terminal biotin-tag and a C-terminal hexahistidine tag, referred to as B6 (Figure 3). Unfortunately, we were unable to purify KCNU1. After binding the protein to cobalt beads via the his-tag, we observed some bands by SDS-PAGE that might correspond to protein of the correct size, but the sample was contaminated with other proteins. We subsequently ran the sample over a size exclusion column, but we were unable to separate the desired protein. The elution fractions from size exclusion contained many proteins of various molecular weights.
For our second purification attempt, we chose B8, which is the same as B6, except a large internal loop of 21 amino acids (S831-N851) was also deleted. We created and tested this design as Leonetti et al. reported successfully purifying this protein construct in the baculovirus/SF9 insect cell system9. Although we observed expression of a protein around the correct size after purification via cobalt beads, our elution was again dirty. As before, size exclusion did not separate out a protein of the correct size, and elution fractions contained many contaminant proteins of various sizes.
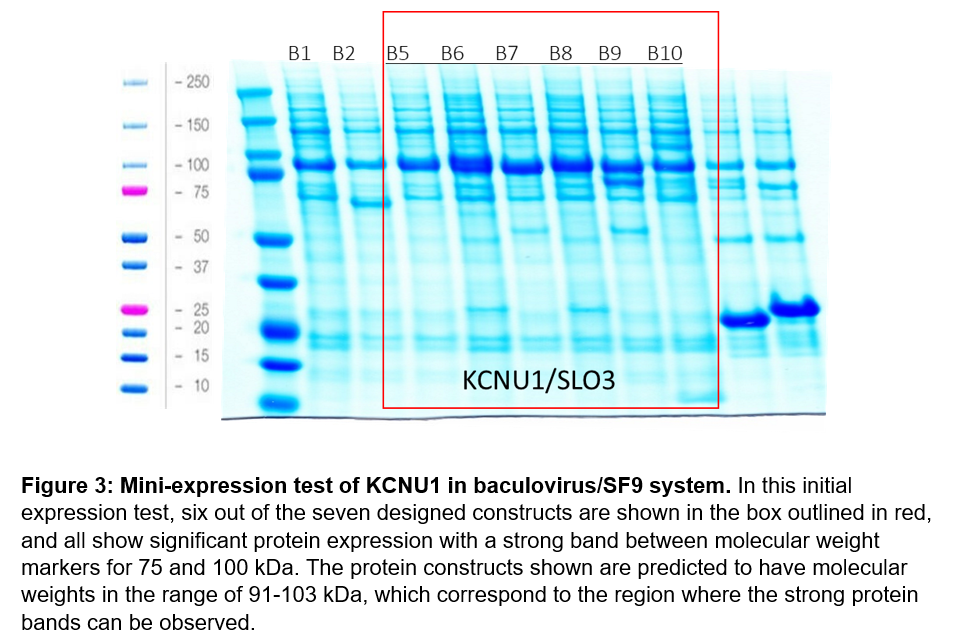
After two unsuccessful purification attempts, we returned to review the files for the protein constructs and discovered an error that may explain why we were unable to purify KCNU1, despite the success of Leonetti et al. Although we had designed these constructs to end at residue 1062, we observed that the cloned constructs were extended at the C-termini past the designed domain boundaries. We have since corrected this error and produced the same 7 protein constructs with corrected domain boundaries.
From the new protein constructs, mini-expression tests indicated that two constructs showed promising expression: KCNU1 G330-S1063 with an N-terminal his-tag, and KCNU1 G330-S1063 with the deleted loop and an N-terminal biotin tag, and a C-terminal his-tag. Since the later most closely mimics the construct used by Leonetti et al., we chose this construct for our first large-scale protein purification. As with the previous two purification attempts for KCNU1, after affinity and size exclusion chromatography we obtained elution fractions with many contaminant proteins (Figure 4).
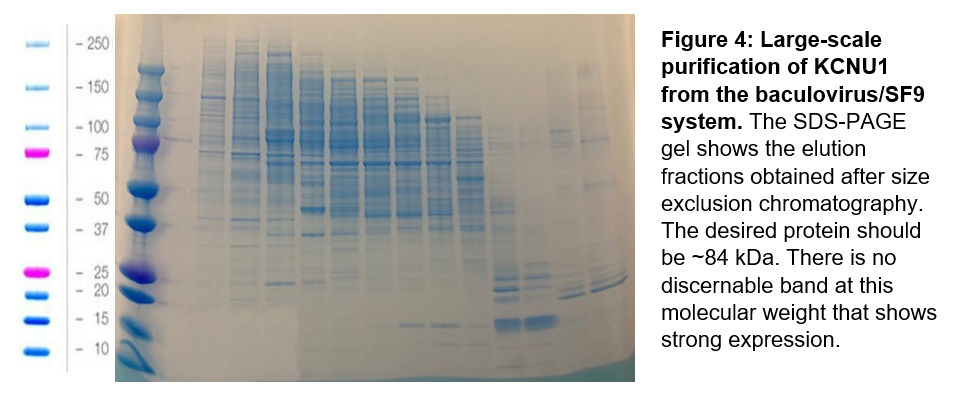
Next steps
As our plans to study KCNU1 are contingent on our ability to express and purify this protein, we must focus our attention on producing a soluble protein construct. We plan to repeat the small-scale expression tests of the corrected KCNU1 constructs to determine if the expression we observed in the initial test can be repeated. Meanwhile, we will generate a protein construct that contains a C-terminal GFP and deca-histidine tag to mimic the construct used by Leonetti et al. Once we determine a construct that is soluble and demonstrates sufficient expression, we will perform a purification that is informed by the protocol used by Leonetti et al. As the authors of this publication were able to concentrate their protein to 5 mg/mL after removing the fusion tags, we are optimistic that we will be able to reproduce these results in our laboratory.
Acknowledgements
I would like to acknowledge Alma Seitova, Ashley Hutchinson, Maria Kutera, and Peter Loppnau for their contributions to clone and express KCNU1 protein constructs. I would like to thank Hong Zeng for her work to purify the initial round of KCNU1 constructs. I would also like to acknowledge Levon Halabelian and Aled Edwards for their input and supervision of this work.
This project is funded by the Bill and Melinda Gates Foundation. The Structural Genomics Consortium is a registered charity (no: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA (aka EMD in Canada and US), Pfizer, Takeda and the Wellcome Trust [106169/ZZ14/Z].
References
(1) Hock, H. The Pill and the College Attainment of American Women and Men. undefined 2007.
(2) Sonfield, A.; Hasstedt, K.; Kavanaugh, M. L.; Anderson, R. The Social and Economic Benefits of Women’s Ability To Determine Whether and When to Have Children. 48.
(3) Troutman, M.; Rafique, S.; Plowden, T. C. Are Higher Unintended Pregnancy Rates among Minorities a Result of Disparate Access to Contraception? Contracept. Reprod. Med. 2020, 5, 16. https://doi.org/10.1186/s40834-020-00118-5.
(4) Bellizzi, S.; Mannava, P.; Nagai, M.; Sobel, H. L. Reasons for Discontinuation of Contraception among Women with a Current Unintended Pregnancy in 36 Low and Middle-Income Countries. Contraception 2020, 101 (1), 26–33. https://doi.org/10.1016/j.contraception.2019.09.006.
(5) Key Achievements. SGC. https://www.thesgc.org/about/key_achievements (accessed 2022-09-15).
(6) Target Enabling Packages (TEPs). SGC. https://www.thesgc.org/tep (accessed 2022-09-15).
(7) Wang, H.; McGoldrick, L. L.; Chung, J.-J. Sperm Ion Channels and Transporters in Male Fertility and Infertility. Nat. Rev. Urol. 2021, 18 (1), 46–66. https://doi.org/10.1038/s41585-020-00390-9.
(8) Liu, R.; Yan, Z.; Fan, Y.; Qu, R.; Chen, B.; Li, B.; Wu, L.; Wu, H.; Mu, J.; Zhao, L.; Wang, W.; Dong, J.; Zeng, Y.; Li, Q.; Wang, L.; Sang, Q.; Zhang, Z.; Kuang, Y. Bi-Allelic Variants in KCNU1 Cause Impaired Acrosome Reactions and Male Infertility. Hum. Reprod. 2022, 37 (7), 1394–1405. https://doi.org/10.1093/humrep/deac102.
(9) Leonetti, M. D.; Yuan, P.; Hsiung, Y.; MacKinnon, R. Functional and Structural Analysis of the Human SLO3 PH- and Voltage-Gated K+ Channel. Proc. Natl. Acad. Sci. 2012, 109 (47), 19274–19279. https://doi.org/10.1073/pnas.1215078109.
(10) Chávez, J. C.; Ferreira, J. J.; Butler, A.; Beltrán, J. L. D. L. V.; Treviño, C. L.; Darszon, A.; Salkoff, L.; Santi, C. M. SLO3 K+ Channels Control Calcium Entry through CATSPER Channels in Sperm *. J. Biol. Chem. 2014, 289 (46), 32266–32275. https://doi.org/10.1074/jbc.M114.607556.
