The Structural Genomics Consortium (SGC) has been funded by the Bill & Melinda Gates Foundation to research Women’s and Children’s Health, focusing on the advancement of drug discovery in reproductive biology and disease, child development, and childhood diseases. The SGC plans to generate protein reagents and chemical probes for several potential drug targets for new, safe and effective non-hormonal contraceptives (NHCs).
PLCζ1 background
One promising protein target for NHCs is phospholipase C zeta 1 (PLCζ1). PLCζ1 is found within the sperm and is known to be a sperm oocyte activation factor (SOAF). PLCζ1 catalyses the hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) into inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG).1,2 Once released, IP3 is then able to bind to its receptors located in specialised compartments of the endoplasmic reticulum (ER) membrane and release intracellular calcium (Ca2+) from the ER. These Ca2+ oscillations are vital for successful fertilisation.1,3
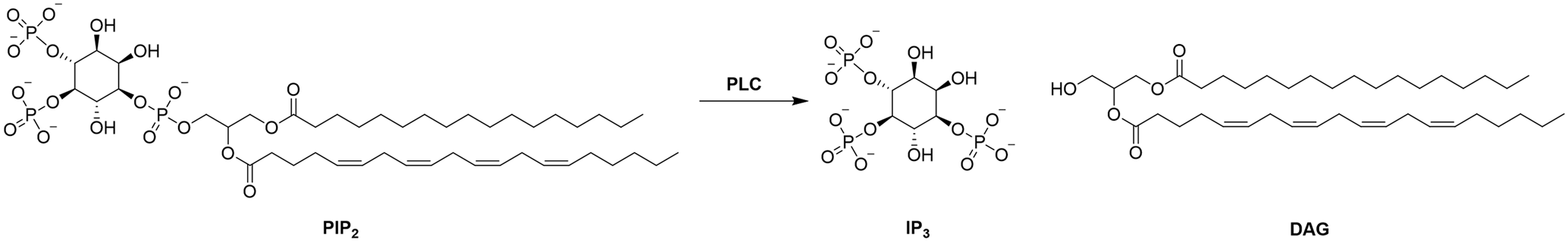
Scheme 1: Hydrolysis of PIP2 into IP3 and DAG.
Protein Production
Madison Edwards has purified MBP-tagged human PLCζ1 from E. coli in a low yield, similar to previously reported expressions.4 However, the protein appears to be in soluble aggregates and contains a chaperone protein contaminant, GroEL, which was not removed through size exclusion chromatography or by washing under recommended conditions.
Expression of human PLCζ1 constructs from mammalian cells was not successful. Dalia Barsyte-Lovejoy and Peter Loppnau expressed codon-optimised human PLCζ1 in mammalian cells, which was visualised by FLAG-tag immunoblot. Expression was low yielding, and the protein was found to have solubility issues. Opher Gileadi tried to improve upon these results by using a different expression vector and exchanging the tag from flag to strep, but expression was still low yielding.
Madison attempted to express PLCζ1 from six animals (chicken, mouse, macaque, cow, pig and horse) from E. coli, but only insoluble protein was obtained. Expression of chicken, mouse and macaque PLCζ1 in a baculovirus/SF9 system was successful, with macfa and mouse found to be active through an IP-one assay5. Chicken PLCζ1 is the only construct that reliably provided high yields of monomeric protein. Chicken PLCζ1 has 62% identity to human PLCζ1 and 68% in the X and Y catalytic domains. Work is ongoing to assess whether chicken PLCζ1 is similar enough to human PLCζ1 for this to be a relevant target to screen for small molecule inhibitors.
Biochemical Assays
Eve Carter is working to establish a biochemical assay with Aldol 518 as a substrate for hydrolysis and Aldol 355 as a fluorescence enhancer, as previously reported for PLCγ1.5 This would provide a cheap and straightforward assay to measure PLCζ1 activity. Two other assays, IP-one5 and XY-696, may also be utilised.
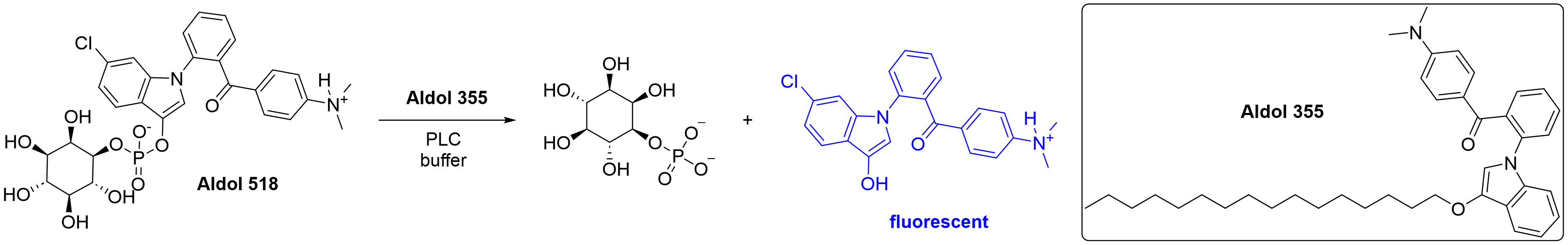
Scheme 2: Hydrolysis of Aldol 518 by PLC can be measured by fluorescence intensity.
Future work
HiBiT Cellular Thermal Shift Assay (CETSA): Dalia Barsyte-Lovejoy, Peter Loppnau and Magdalena Szewczyk are working on setting up a CETSA assay for human and chicken PLCζ1. This would provide an orthogonal assay to confirm any proposed binders/inhibitors to the protein.
DNA Encoded Library (DEL) screen: If we confirm that chicken PLCζ1 is a suitable orthologue to investigate, we will send this for a DEL screen. This will provide a starting point for the identification of chemical probes to investigate PLCζ1.
Crystallography: We will attempt to crystalise PLCζ1 to further the understanding of this protein and assist with the discovery of small molecule binders. So far, the only PLC crystal structure is that of PLCδ1.7
Antibodies: In collaboration with YCharOS and Thermo Fisher Scientific, we are working towards generating a PLCζ1 antibody.
References
(1) Thanassoulas, A.; Swann, K.; Lai, F. A.; Nomikos, M. The Structure and Function Relationship of Sperm PLCZ1. Reproduction 2022, 164 (1), F1–F8. https://doi.org/10.1530/REP-21-0477.
(2) Nomikos, M.; Kashir, J.; Lai, F. A. The Role and Mechanism of Action of Sperm PLC-Zeta in Mammalian Fertilisation. Biochem. J. 2017, 474 (21), 3659–3673. https://doi.org/10.1042/BCJ20160521.
(3) Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F. A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8 (28), 1–9. https://doi.org/10.3389/fcell.2020.00028.
(4) Nomikos, M.; Stamatiadis, P.; Sanders, J. R.; Beck, K.; Calver, B. L.; Buntwal, L.; Lofty, M.; Sideratou, Z.; Swann, K.; Lai, F. A. Male Infertility-Linked Point Mutation Reveals a Vital Binding Role for the C2 Domain of Sperm PLC ζ. Biochem. J. 2017, 474, 1003–1016. https://doi.org/10.1042/BCJ20161057.
(5) Le Huray, K. I. P.; Bunney, T. D.; Pinotsis, N.; Kalli, A. C.; Katan, M. Characterization of the Membrane Interactions of Phospholipase Cγ Reveals Key Features of the Active Enzyme. Sci. Adv. 2022, 8 (25), 1–16. https://doi.org/10.1126/sciadv.abp9688.
(6) Huang, W.; Wang, X.; Endo-Streeter, S.; Barrett, M.; Waybright, J.; Wohlfeld, C.; Hajicek, N.; Harden, T. K.; Sondek, J.; Zhang, Q. A Membrane-Associated , Fluorogenic Reporter for Mammalian Phospholipase C Isozymes. J. Biol. Chem. 2018, 293 (5), 1728–1735. https://doi.org/10.1074/jbc.RA117.000926.
(7) Essen, L.-O.; Perisic, O.; Cheungt, R.; Katant, M.; Williams, R. L. Crystal Structure of a Mammalian Phosphoinositide-Specific Phospholipase C Delta. Nature 1996, 380, 595–602. https://doi.org/10.1038/380595a0.
