hFetuin B(1-382)-Fc-6xHis and hOvastacin(1-431)-twinStrep purification: Nickel & Strep-Tactin®T 4Flow® high capacity FPLC column
For relevant background please see relevant page: Assessing Ovastacin and Fetuin B as a Non-Hormonal Contraceptive Targets (https://openlabnotebooks.org/assessing-ovastacin-an-fetuin-b-as-a-non-hormonal-contraceptive-targets/)
Donor Plasmid:
pFastBac-Dual vector was used for the dual expression of hOvastacin-twinStrep (driven by polyhedrin promoter) and hFetuin B-Fc-6xHis (driven by p10 promoter).
Recombinant bacmid DNA preparation:
- DH10Bac™ coli was used for the production of the recombinant bacmid (1ng donor plasmid to 100ul DH10Bac™ competent cells);
- The LB medium containing kanamycin, gentamicin, tetracycline, X-β-Gal, and IPTG for Blue-White screening was used to pre-select the coli colonies with transposed bacmid, and the white colonies were then confirmed by re-spreading on the fresh LB medium containing the above mentioned antibiotics, X-β-Gal, and IPTG;
50 ug/ml kanamycin
7 ug/ml gentamicin
10 ug/ml tetracycline
200 ug/ml X-β-Gal
40 ug/ml IPTG
- Double-check the pre-selected white colonies by performing colony PCR using pUC/M13 Forward and Reverse primers (the PCR amplified DNA band from the negative colonies should be around 350bp, while the DNA band from the positive colonies should be 350bp plus the size of the DNA inserted, which is around 2kb);
pUC/M13 Forward primer: 5′-CCCAGTCACGACGTTGTAAAACG-3′
pUC/M13 Reverse primer: 5′-AGCGGATAACAATTTCACACAGG-3′
- High-pure extraction of the recombinant bacmid DNA from the positive colony identified.
Baculovirus preparation:
- Pre-incubate the insect cell sf9 (in Insect-XPRESS protein-free cell medium with L-glutamine), make sure the cells are healthy with greater than 95% viability and are growing in the logarithmic phase with a density of 2×10^6 cells/ml before proceeding to transfection;
- Prepare 2ml of the insect cells (8×10^5 cells/ml) in a 6-well tissue culture plate, transfect the cells with 1ug of the purified recombinant bacmid DNA using Cellfectin® II Reagent;
- Incubate the transfected insect cells at 27°C for 72 hours, and harvest the supernatant to fresh 15 ml snap-cap tubes, centrifuge the cells at 800g x 5min to collect the supernatant (P1 virus stock, around 2ml). Store the P1 virus stock at 4°C, protect from light;
- Amplify the P1 viral stock in suspension culture at 2×10^6 cells/ml to get a higher titer of P2 viral stock (50ml medium with 2ml P1 virus stock in a 1L flask). Make sure that the cells used are healthy, and have >95% viability before proceeding to infection. Incubate the cells in a shaker with a setting of 27°C, 170rpm for 72h. Centrifuge the transfected cells at 800g x 5min to collect the supernatant (P2 viral stock). Store the virus stock at 4°C (protect from light) for recombinant protein production. Amplify the P2 virus to get a large volume of P3 virus if necessary.
Co-expression and purification of hFetuin B-Fc-6xHis and hOvastacin-twinStrep:
- To express the recombinant protein, infect 2L of sf9 cells with Baculovirus (each of the 500ml sf9 suspension cell cultures with 12ml P2 or P3 baculovirus). Make sure the cells are healthy with greater than 95% viability and are growing in the logarithmic phase with a density of 2×10^6 cells/ml. Incubate the infected cells in a shaker with a setting of 27°C, 170rpm for 72h;
- Centrifuge the cell culture at 1000g x 20min, RT, collect the supernatant (hFetuin B-Fc-6xHis and hOvastacin-twinStrep are secreted proteins) to a beaker, add solid ammonium sulfate slowly with gentle agitation (allow to dissolve before adding more solid, try to prevent foaming) to get a final 60% saturated buffer solution. Let the protein precipitate in ammonium sulfate buffer at 4°C overnight with gentle agitation;
- Centrifuge the overnight precipitated buffer at 17000g for 2h, 4°C, carefully discard the supernatant and leave the precipitation, dissolve the precipitation with 200ml (1/10 volume relative to the cell culture used) of loading buffer;
- Dialysis the dissolved precipitation buffer solution in 2L loading buffer at 4°C for 4h with gentle agitation. An extra centrifuge step (8000g for 20min at 4°C) is necessary if the solution is turbid, otherwise not necessary;
- Ni-IMAC purification of protein:
- Rinse the Nickel (Ni) beads with loading buffer (pre-incubate the loading buffer at 4°C);
- For each of the 40ml protein solution from the above step 4), add 1ml of Ni beads in a 50ml falcon tube, incubate at a shaker with gentle shake at 4°C overnight;
- Transfer the protein solution with Ni beads to the IMAC column at 4°C, collect the flow through (FT);
- Wash the beads with 200ml of wash buffer, collect the wash buffer for SDS-PAGE analysis;
- Elute with 10ml of elution buffer, and concentrate the elute to around 5ml;
- Take 2ug protein for mass spectrometry (MS) analysis to check the protein molecular weight. Run gel filtration (GF) of the 5ml elute (filtered with 0.22um filter before GF) with S75 in 500ml of chromatography buffer;
- Run SDS-PAGE of FT, wash buffer, elute and the proteins with peak from the GF;
- Collect the protein after GF, concentrate the protein, aliquots into small volume and flash freeze with liquid N2, store at -80°C.
Loading buffer used:
20mM Tris-HCl, 20mM imidazole, 500mM NaCl, 1mM TCEP, 10% glycerol
Wash buffer used:
20mM Tris-HCl, 30mM imidazole, 500mM NaCl, 1mM TCEP, 10% glycerol
Elution buffer used:
20mM Tris-HCl, 300mM imidazole, 100mM NaCl, 0.5mM TCEP, 10% glycerol
Chromatography buffer used:
20mM Tris-HCl, 100mM NaCl, 0.5mM TCEP, 10% glycerol
- Strep-Tactin®T 4Flow® high capacity FPLC column (2-5028-001) purification of protein:
- Equilibrate the column (5ml bed volume) with 25ml (5 CVs) of wash buffer (pre-incubated at 4°C), the flow rate should be in the range of 1-3 ml/min;
- Apply the protein solution from the above step 4) into the Strep-Tactin®T 4Flow® column, collect the flow through (FT);
- Wash the column with 40ml (8 CVs) of wash buffer, collect the wash buffer;
- Elute the protein with 25ml (5 CVs) elution buffer, collect the elute, concentrate the elute;
- Run SDS-PAGE with FT, wash buffer, elute;
- Regenerate the Strep-Tactin®T 4Flow® column with 75ml (15 CVs) of 3M MgCl2, wash with 25ml (5CVs) of wash buffer, store the column at 4°C;
- Aliquots the concentrated protein into a small volume and flash freeze with liquid N2, store at -80°C.
Wash buffer used:
100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1mM TCEP, 10% glycerol
Elution buffer used:
100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 50 mM biotin, 0.5mM TCEP, 10% glycerol
Ni-IMAC purification of hFetuin B-Fc-6xHis and hOvastacin-twinStrep protein complex:
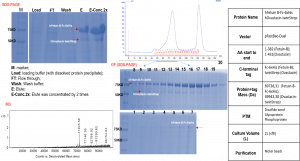
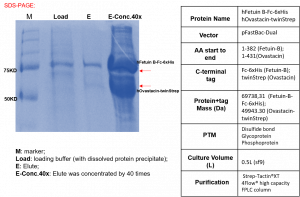
Strep-Tactin®T 4Flow® high capacity FPLC column purification of hFetuin B-Fc-6xHis and hOvastacin-twinStrep protein complex:
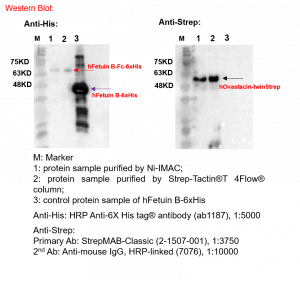
Western Blot to confirm hFetuin B-Fc-6xHis and hOvastacin-twinStrep:
This project is funded by the Bill and Melinda Gates Foundation. The Structural Genomics Consortium is a registered charity (no: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], the EU and EFPIA through the Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA (aka EMD in Canada and US), Pfizer, Takeda and the Wellcome Trust [106169/ZZ14/Z].
