Background
The SCF (SKP1-CUL1-F-box protein) E3 ubiquitin ligase is a complex of proteins that helps facilitate the ubiquitination of substrates for targeted proteasomal degradation. FBXW7 (F-box/WD repeat-containing protein 7) is an F-box protein component of the SCF complex and is involved in substrate recognition. The F-box domain tethers FBXW7 to the SCF complex by interacting with the SKP1 component while the WD40 repeats are involved in substrate binding. FBXW7 recognizes and binds its substrates at phosphorylated sites or phosphodegrons and recruits them to the SCF complex for subsequent ubiquitination and degradation (PMID:17434132). A variety of substrates exist for FBXW7, many of which are potentially oncogenic, including cyclin E1, MYC, JUN, and NOTCH (PMID: 11565034, 15103331, 14739463, 29149593). The targeted degradation of growth-promoting factors by FBXW7 suggests it likely plays an important role as a tumor suppressor. FBXW7 Arg→Cys mutation at position 465 hinders its ability to interact with SKP1 and thus the SCF complex; this mutation is associated with acute lymphoblastic leukemia (PMID: 11565033, 28007894).
Assay validation
Assay measuring the interaction of FBXW7 with cyclin E1 was developed using NanoBRET technology – a proximity-based assay that can detect protein interactions by measuring energy transfer from a bioluminescent protein donor (NanoLuc, NL) to a fluorescent protein acceptor (fluorescent ligand bound to HaloTag, HT). In the beginning, we determined the optimal tag orientation of donor constructs: N or C-terminally NL-tagged cyclin E1 and acceptor constructs: N or C-terminally HT- tagged FBXW7 in HEK293T cells. NanoLuc and halo tag proteins, expressed alone, were used as controls to determine background signal. The best results were obtained with C-terminally NL-tagged cyclin E1 and C-terminally HT-tagged FBXW7 (Fig.1). The assay was further validated with a binding deficient FBXW7 mutant (R465E, R505E) designed by Matthieu Schapira. R465E, R505E double mutation resulted in a significant decrease in the interaction of FBXW7 with cyclin E1 (Fig.2).
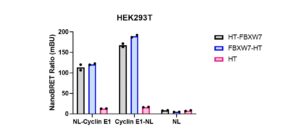
Fig.1. FBXW7 interaction with cyclin E1 – NanoBRET assay. HEK293T cells were co-transfected with C- or N-terminally NanoLuc (NL)-tagged cyclin E1 or NL alone and C- or N-terminally Halo-tagged (HT) FBXW7 or HT alone for 24 h. The interaction was measured using NanoBRET assay. The results are MEAN of 2 technical replicates.

Fig.2. R465E, R505E double mutation decreases the interaction of FBXW7 with cyclin E1. HEK293T cells were co-transfected with C-terminally Halo-tagged (HT) FBXW7 (WT or R465E/R505E or HT and C-terminally NanoLuc (NL)-tagged cyclin E1 for 24 h. The interaction was measured using NanoBRET assay. The results are MEAN of 2 technical replicates.
Please go to Zenodo for experimental details.
Acknowledgments:
Matthieu Schapira – mutant design, Aidan Levinsky – help with mutagenesis

I wonder why did you prefer R465E/R505E mutations rather than R465C/R505C mutations?
Thank you!