Ubiquitination can mark proteins for degradation, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitin specific proteases (USPs) are the largest family of deubiquitinase enzymes. USP5 disassembles unanchored ubiquitin chains. This prevents the accumulation of ubiquitin chains which would otherwise compete against ubiquitinated proteins for degradation as well as replenishes the monoubiquitin pool [1]. In addition to the catalytic domain, USP5, contains a zinc-finger ubiquitin binding domain (Zf-UBD) which recognizes the C-terminal of ubiquitin and allosterically activates the catalytic site. USPs have been targeted by the drug discovery community for over a decade for the treatment of diseases such as cancer and neurodegeneration, but, with a few, rare exceptions, efforts to develop catalytic inhibitors have failed thus far. The goal of this research project is to develop ZnF-UBD chemical probes (potent, selective, cell-active small molecule inhibitors), to see if pharmacologically targeting the non-catalytic domain of USP5 is a valid therapeutic strategy.
A number of reports show that USP5 is a promising therapeutic target. Depletion of USP5 increases the level of p53, a major tumor suppressor, indicating that USP5 is a potential target for cancer therapy [2]. Recent data also shows that USP5 is essential for the proliferation and survival of pancreatic cancer cells [3]. In both cases, the role of the Zf-UBD of USP5 is unclear, and could be interrogated with selective inhibitors. Additionally, the interaction of the Zf-UBD of USP5 with a voltage-dependent calcium channel leads to inflammatory and neuropathic pain, suggesting that small molecules inhibiting this interaction could have pain relief effects [4][5](Figure 1).
This project encompasses a variety of disciplines ranging from molecular and structural biology to computational chemistry. I will approach this project by first expressing and purifying the USP5 Zf-UBD protein domain, and developing biophysical assays to characterize its interaction with small-molecule inhibitors. I will also solve high-resolution, 3-dimensional crystal structures of USP5, and use computational approaches such as docking and Free Energy Perturbation (FEP) to design and optimize small molecule inhibitors.
Combining these approaches, I hope to develop chemical tools that can be used to evaluate the Zf-UBD of USP5 as a target for cancer therapy and neurological disorders.
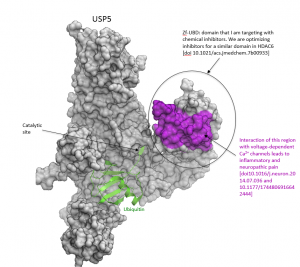
Figure 1. USP5 structure
[1] Komander, D., et al. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol, 2009. 10, 550–563.
[2] Dayal, S., et al. Supression of the Deubiquitinating Enzyme USP5 Causes the Accumulation of Unanchored Polyubiquitin and the Activation of p53. JBC, 2008. 284, 5030-5041.
[3] Kaistha, P. B., et al. The deubiquitinating enzyme USP5 promotes pancreatic cancer via modulating cell cycle regulators. Oncotarget, 2017. 8 (39), 66215-66225.
[4] Garcia-Caballero, A., et al. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing Cav3.2 channel activity. Neuron, 2014. 83(5), 1144-1158.
[5] Garcia-Caballero, A., et al. A cell-permeant peptide corresponding to the cUBP domain of USP5 reverses inflammatory and neuropathic pain. Mol Pain, 2016. 12, 1744806916642444.
