For the past few days, I have been working on optimizing a fluorescence polarization (FP) displacement assay for USP5 Zf-UBD. This assay will be used to test if small molecules can displace a fluorescence-labelled ubiquitin peptide from USP5.
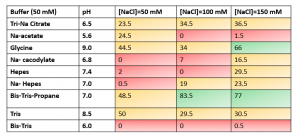
I started with screening different assay conditions with a small set of buffers. The method and data from this experiment can be found on Zenodo. The aim was to find buffer conditions that gave the best signal to noise ratio. In the table below, the buffer conditions that gave the best FP range can be seen in green.
Next, I tested different USP5 Zf-UBD concentrations with a FITC-labelled C-terminal RLRGG-peptide. To achieve 100% FP, you need around ~80 µM of protein.
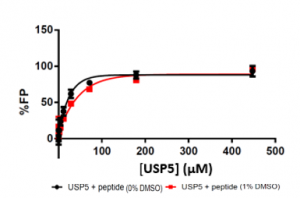
The protein concentration is quite high, so I’ll definitely need to purify loads of protein for this assay. Because the USP5 concentration is high, I will need to screen compounds at high concentrations, and will need a more sensitive assay to validate screening hits. Stay tuned!
