In my last posts, I have constructed a pair of split-SCN1A constructs (split- SCN1A-GFP(1-10) and SCN1A-GFP(11)). Here, I tested the functionality of these constructs by looking at whether these split- constructs reconstitute into a full-length SCN1A and generate a fluorescent signal when co-transfected in HEK293 cells, and if so, do the reconstituted SCN1A (with an internal GFP tag) localize correctly at the plasma membrane.
Below are my imaging results. The detailed experimental procedure can be found at https://zenodo.org/record/1490851#.W_G_bpNKh3g
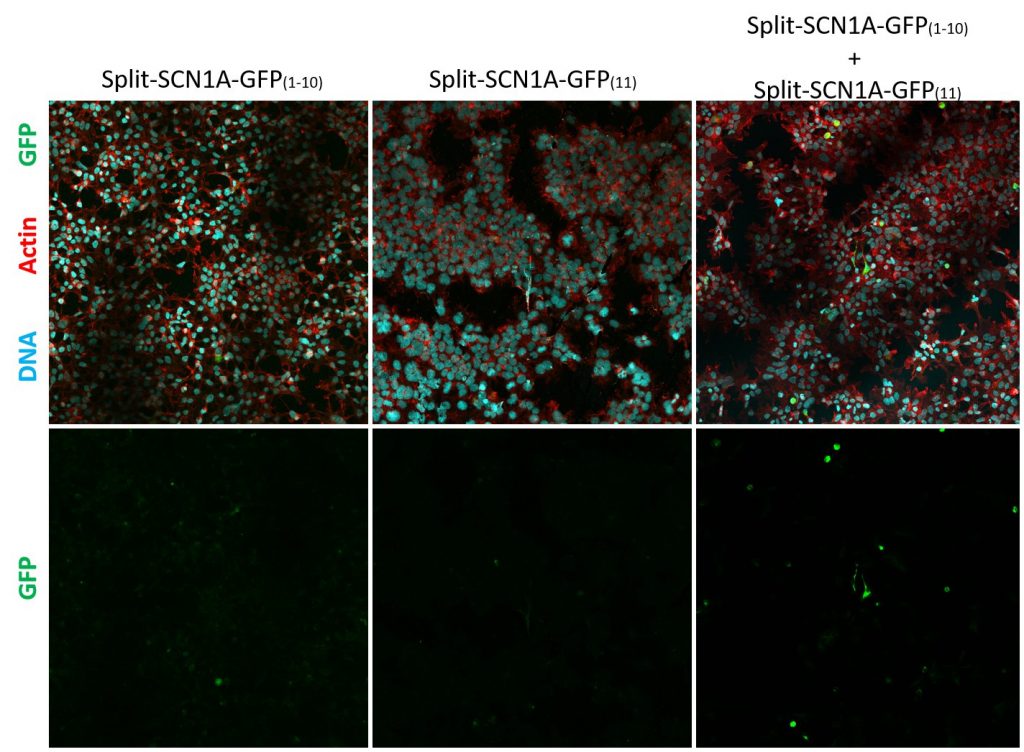
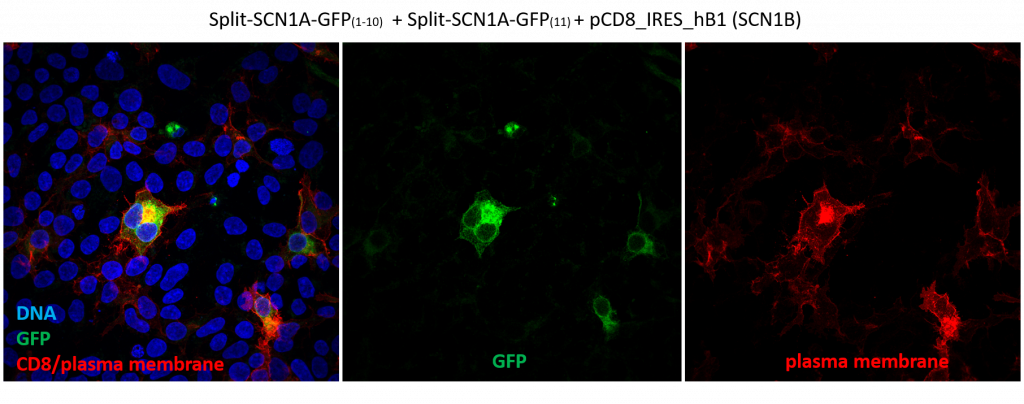
In short, these split-constructs managed to find each other and reconstitute a GFP signal in HEK293 cells. However, the GFP signals do not overlap with cell cortex or plasma membrane markers (cell cortex marked by cortical actin staining and plasma membrane marked by CD8, a transmembrane glycoprotein.) The distribution of reconstituted SCN1A-GFP signals resembles misfolded proteins that are trapped in the ER. Co-expression of SCN1B, a beta subunit which is thought to promote sodium channel trafficking, does not seem to improve the localization of reconstituted SCN1A-GFP.
There are several caveats with the design of these constructs which might have affected folding and trafficking of reconstituted SCN1A: 1) instead of using the canonical SCN1A cDNA sequence (which is unstable and prone to mutagenize when propagated in E. coli), a codon-optimized SCN1A cDNA was used as a template in cloning these constructs and 2) the reconstituted protein has a foreign GFP sequence which might have interfered with SCN1A trafficking. Moving forward, I will revisit and examine the localization of codon optimized SCN1A in HEK293 cells while continue constructing the rest of the split-intein constructs.
