We have received some very interesting biological data for our CaMKK2 inhibitor series (furopyridine, Azaindole and quinoline) and I will be sharing the data in the coming weeks, but before that, I wanted to share my synthetic efforts towards our quinoline series.
Initial synthesis of quinoline analogs:
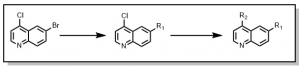
Starting with 6-Bromo-4-chloroquinoline, a two (2) step synthetic scheme was devised where the bromine (Br) synthetic handle was first substituted (with an appropriate boronic acid/ester), followed by the chlorine (Cl) , Figure 1, after testing many different reaction conditions for selectivity.
Figure 1: Synthesis of quinoline series
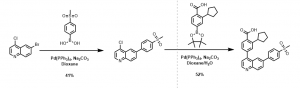
A number of analogs were prepared using the above synthetic strategy, for example, the synthesis of 2-cyclopentyl-4-(6-(4-(methylsulfonyl)phenyl)quinolin-4-yl)benzoic acid was achieved using the above described method, Scheme 1.
Scheme 1: Synthesis of quinoline analog
Although this synthetic strategy led to the synthesis of some analogs, it was not without challenges. For instance, selectivity for the Br was not always achieved as disubstituted products were common in some cases due to the activated 4-position of the Cl.
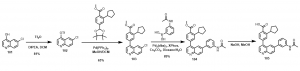
In order to overcome these challenges and to allow for rapid synthesis of different analogs over a short period of time, a strategy to first install the cyclopentyl derivative was sort after. In the synthetic scheme below, Scheme 2, 6-chloroquinolin-4-ol, 101, was treated with triflic anhydride in the presence of DIPEA in DCM to obtain 6-chloroquinolin-4-yl trifluoromethanesulfonate, 102. This compound was able to undergo the Suzuki cross coupling reaction to obtain compound 103. With this compound (103) in hand, a number of different analogs are being generated in our search for active CaMKK2 chemical probes, for example compound 105, Scheme 2.
Scheme 2: Synthesis of quinoline analog via new route
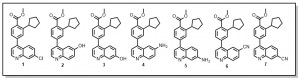
We are also looking to exploring the 7-position of the quinoline with similar substitutions to the 6-position (Figure 2), quinoline derivative 1. Other anticipated analogs will include O-alkylations (quinoline derivatives 2 and 3), N-alkylations or peptide couplings or Hartwig-Buchwald aminations (quinolines derivatives 4 and 5) etc. Details of such reactions will be communicated in due course.
Figure 2: Exploration of other quinoline analogs