The line up of inhibitor series (hinge binders) for CaMKK2 currently being pursued include: furopyridine, azaindole (pyrrolopyridine), quinoline, triazolopyridazine, imidazopyrazine, pyrazolopyrimidine. Figure 1.
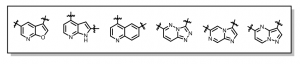
Figure 1: CaMKK2 hinge binders
As part of our structure and activity relationships (SAR), we continue to build diversity on the pyridine ring for the furopyridine series, and new compounds added to the set are provided below in Figure 2.
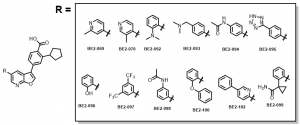
Figure 2: Additional Furopyridine Analogs
The Azaindoles (Pyrrolopyridines)
Below, I provide the synthetic efforts towards the azaindole (pyrrolopyridines) series, (Bioorg. Med. Chem. Lett. 28 (2018), 1958-1963). The goal is to evaluate what effect changes in the acid functionality with/without the isopropyl substituent would have on the activity of this series (Figure 3). It is hoped that any observed trend in activity, solubility, permeability etc will guide the discovery efforts of this inhibitor series for CaMKK2.
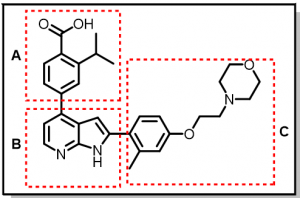
Figure 3: Azaindole Analog
Synthesis of 2-isopropyl-4-(2-(2-methyl-4-(2-morpholinoethoxy)phenyl)-1H-pyrrolo[2,3-b]pyridin-4-yl)benzoic acid, BE2-041:
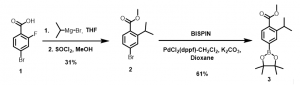
a. Synthesis of ‘A‘ portion (Figure 3) of the molecule (Scheme 1): Compound 1 was able to undergo the Grignard reaction to install the isopropyl substituent in compound 2, which was then converted to the corresponding boronic ester derivative, 3.
Scheme 1: Synthesis of
b. Synthesis of ‘C’ portion (Figure 3) of the molecule (Scheme 2): Compound 6 was obtained via the Mitsunobu reaction and subsequently converted to the corresponding boronic ester derivative, 7.
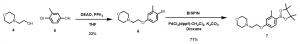
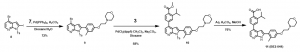
c. Putting it all together: Synthesis of BE2-041 (Scheme 3): Compound 11 (BE2-041) was obtained via a series of Suzuki reaction methodologies and a final deprotection reaction to achieve the target compound.
Completed compounds compounds of the azaindole series are provided in Figure 4.
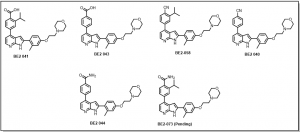
Figure 4: Completed Azaindole Series
