Having previously purified BMPR1A (Alk3) and BMPR1B (Alk6), I spent some time purifying FKBP12 to form complexes with each of the receptors. FKBP12 binds to BMPR1A and BMPR1B via a similar GS domain to that seen in ACVR1, on the N-terminal side of the kinase domain and may help stabilize the structures and thus help it to crystallise.
I purified the complex using size exclusion chromatography and confirmed complex purity by SDS-Gel electrophoresis.
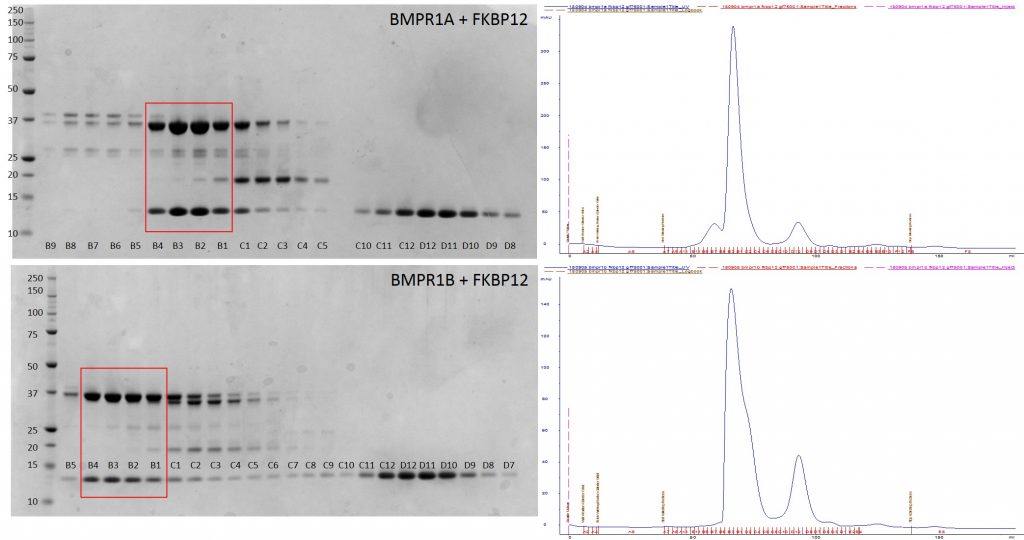
Purification of BMPR1A + FKBP12 and BMPR1B + FKBP12 complexes by gel filtration. SDS PAGE gel samples on left. AKTA trace shown on right. Fractions of purified complex used for crystallography shown boxed in red.
Having purified the complex, the proteins were concentrated down to 13.3mg/ml and 7.5mg/ml respectively. I added one of three compounds at 0.7mM to aliquots of each complex, spun down the samples and set up crystal plates using a selection of coarse chemical screens as shown below. The limited amount of BMPR1B limited the compounds and screens that could be tested.
| M4K2009 | M4K3003 | M4K3007 | |
| BMPR1A + FKBP12 | HCS3/HIN3/BCS/JCSG7 | HCS3/HIN3/BCS/JCSG7 | HCS3/HIN3/BCS/JCSG7 |
| BMPR1B + FKBP12 | HCS3/HIN3/BCS | HCS3/HIN3/BCS/JCSG7 |
Summary table of coarse screens set up for each compound and complex. Screens were then split into two group incubated at either 20 °C and 4°C.
After about a week I checked back on both these screens and on several others that I’d previously set up and talked about here. I am very pleased to report that there were several crystals that were worth mounting!
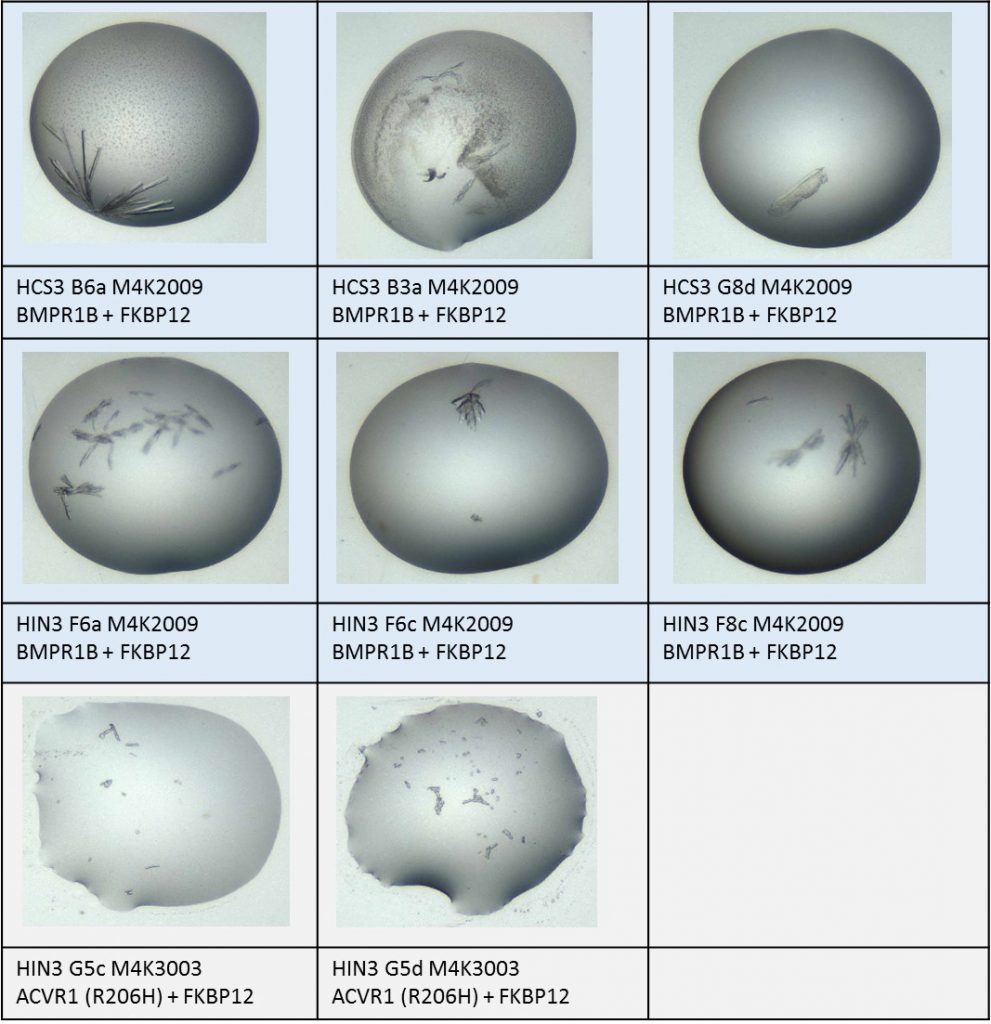
Summary of xtals mounted of either BMPR1B complex or ACVR1 complex with M4K compounds.
Now I’m waiting for a trip to Diamond to see if and how well these diffract. In a stroke of incredible luck, our next trip was meant to be on the 3rd of October but another group cancelled their time and we got offered it instead! So my crystals will be tested this coming Sunday! Watch this space!
For more details please check out the Zenodo post.
