Continuing with the line of delivering a chemical probe for DRAK2/STK17B kinase, we decided to revise the procedure to synthesize analogs with a thieno[2,3-d]pyrimidine core, which are intended to be used as negative control in our DRAK2 inhibition study.
We shortened the synthesis of these pyrimidines to 2 steps from the starting material 6-bromo-4-chlorothieno[2,3-d]pyrimidine 1 and mercaptoacetic acid 2, Figure 1. Here, we eliminated a previous step involving the saponification of an ester moiety to get to the carboxylic acid 3, which is obtained in quantitative yield and easily purified by rinsing with water.
Using this shorter route, we prepared a few analogs AP-90, AP-94, AP-262-264, which were recently screened as DRAK2 inhibitors, showing all of them cell potency >10,000 nM; in other words, they are inactive against DRAK2 and because of their structural similarity with the active compounds in this series, they can be used as negative control for future experiments and probe development.
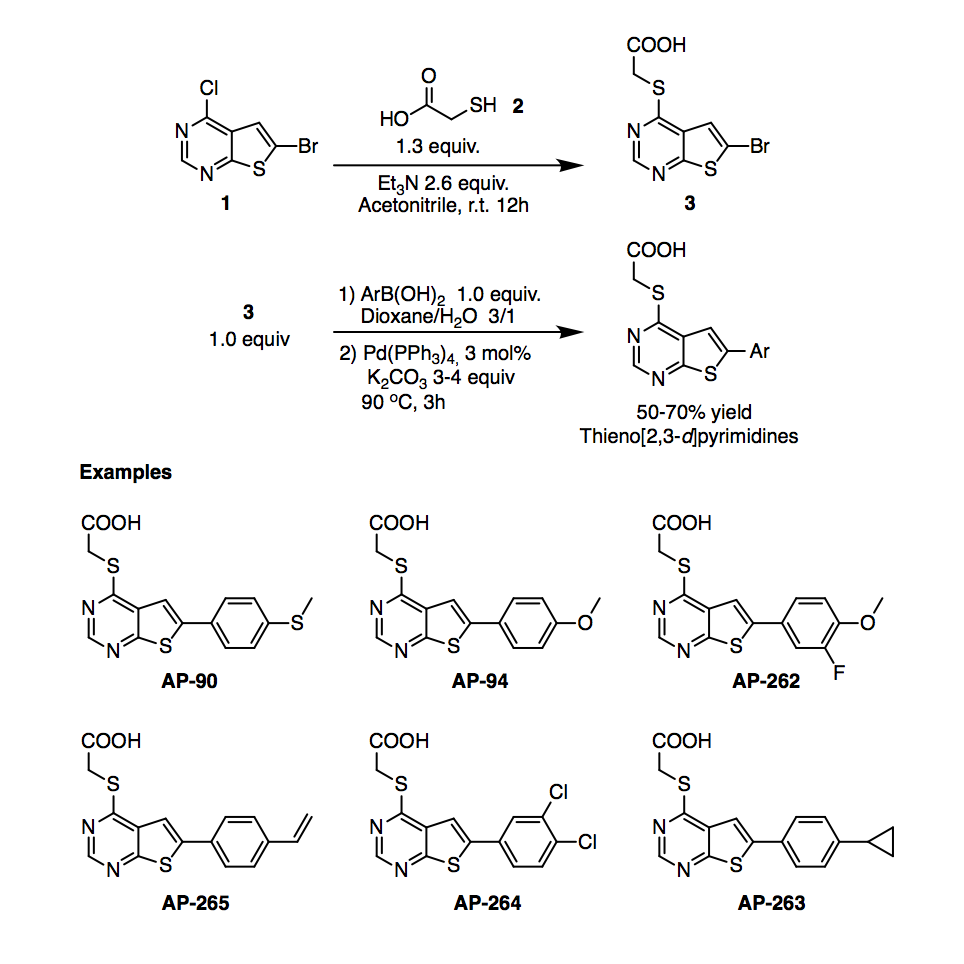
Figure 1. Synthesis of thieno[2,3-d]pyrimidine derivatives.
