Preparation of GW807982X analogues can be find in Tavares’s patent from 2004 (WO2004/35588, 2004, A1). This involves a 4‑step synthesis starting from commercially available 3,6-dichloropyridazine (see previous blog, https://openlabnotebooks.org/project-overview-work-towards-a-clk-probe). From my experience in the lab, formation of the highly nitrogenated pyrazolopyridazine 3 is not a trivial step. This is probably because the formation of the charged aminopyridazinium 2 is not always successful and therefore there is no further reaction to give compound 3. Also, isolated yields after purification are generally low, due to the poor solubility of 3 in organic solvents.
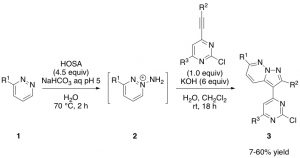
Aminopyridazinium 2 is formed in situ by reaction of pyridazine 1 with hydroxylamine-O-sulfonic acid, also known as HOSA. HOSA is a white solid and water soluble inorganic compound, which is it used as a reagent for the introduction of amine groups (-NH2). HOSA has dual reactivity: under basic conditions it reacts as a nucleophile, whereas under neutral conditions it reacts as an electrophile. Due to this the reaction conditions must be well established and pH corrections have to be done.
Once aminopyridazinium 2 is formed, desired core 3 is isolated by a formal [3+2] cycloaddition with the appropriate alkyne under basic conditions. Work-up and purification of these cores gave a bit of trouble due to the poor solubility in organic solvents. But luckily there is a trick that helps! Interestingly, separation of 3 from undesired side products can be tracked by highlighting the column with a UV light because heterocycle 3 glows as bright blue colour (Picture 1). This makes the chemist’s life easier, although spotting fractions by TLC is always needed to double check (Picture 2).
Fluorescent molecules usually have rigid structures with delocalized electrons, and so this is the reason the compounds are fluorescent under UV light. There are a lot of everyday materials that glow under a UV light, like the bitter flavoring of tonic water due to the presence of quinine or antifreeze liquids. They absorb the UV light and re-emit it almost instantaneously showing glow under dark light even when they are in solution (Picture 3).

Picture 1. Column cartridge highlighted with a UV light to track core 3.
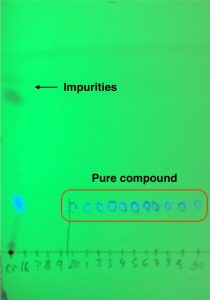
Picture 2. TLC which combines crude material on the left and collected pure fractions.

Picture 3. NMR sample in solution of pure compound (right tube) under UV-light.
Table below shows all the different building blocks I have prepared using this methodology. Some yields were improved after several attempts getting up to a 60% yield.
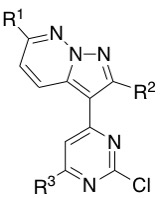
| R1 | R2 | R3 | Yield / % |
| H | H | H | 58 |
| OEt | H | H | 45 |
| OMe | H | H | 52 |
| OCH2CF3 | H | H | 7 |
| OiPr | H | H | 22 |
| OEt | Propyl | H | 59 |
| OEt | Cyclopropyl | H | 60 |
| OEt | THP | H | 48 |
| H | Propyl | H | 30 |
| H | H | Me | 47 |
If you have any questions/comments or want to know more about experimental details, please feel free to contact me.

Great pictures from in the lab and great data!
Did you see any increase or decrease in fluorescence when R1 and R2 substituents were varied?
No, I did not see any increase or decrease. Just the same shiny fluorescent in all the analogues I isolate.
I love the tonic water trivia!