Hi
Here are the details of the assay I developed to measure interaction between USP5 and ubiquitin in cells.
Ubiquitin specific peptidase 5 (USP5) belongs to the largest ubiquitin-specific protease (USP) subfamily and has a zinc finger at its N-terminal portion and two ubiquitin-associated domains in the large catalytic region that mediate polyubiquitin binding (PMID:28923280, PMID:18482987). Structural and biochemical analysis indicated that USP5 cleaved preferentially branched ubiquitins, including unanchored polyubiquitin chains (PMID:19098288). The zinc finger ubiquitin-binding domain (Zf-UBD) recognizes the proximal C-terminal di-glycine motif of ubiquitin (Fig.1).
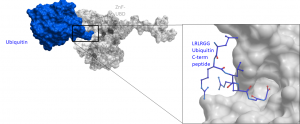
Fig.1. The Zf-UBD recognizes the proximal C-terminal di-glycine motif of ubiquitin. Crystal structure of ubiquitin (blue) binding to USP5 zinc finger ubiquitin-binding domain (grey). Data generated by Mandeep Mann.
Assay measuring the interaction of USP5 to ubiquitin was developed using NanoBRET technology – a proximity-based assay that can detect protein interactions by measuring energy transfer from a bioluminescent protein donor to a fluorescent protein acceptor. We tested different amounts of the donor (N-terminally or C-terminally NanoLuc® tagged full-length USP5) and acceptor (N-terminally HaloTag®Fusion-tagged wild type ubiquitin and mutant lacking RGG motif) constructs in two different cell lines (MCF7 and HEK293). Consistently with in vitro data ubiquitin lacking the last three C-terminal RGG showed lower interaction compared to wild type ubiquitin (Fig.2). The best WT to Mutant ratio was observed in MCF7 transfected with 0.02 µg of HaloTag®Fusion-tagged -Ub/UbdelRGG and 0.001 µg of N- terminally NanoLuc® -tagged-USP5 per 96-well (Fig.2A).
Please see the experimental details on Zenodo.
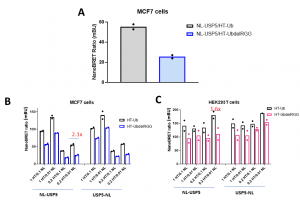
Fig.2. USP5 interaction with ubiquitin and delRGG ubiquitin mutant – NanoBRET assay. MCF7 or HEK293T cells were co-transfected with different amounts of N- or C-terminally NL-tagged USP5 and N-terminally HT-tagged Ub or UbdelRGG mutant, as indicated (per 96/well). The signal was red 48 h post transfection. A. Best WT to mutant ratio was observed in MCF7 transfected with 0.02 µg of HT-Ub/UbdelRGG and 0.001 µg of N- terminally NL-tagged USP5. B, C. Mean corrected NanoBRET ratio (mBU). The condition with the best WT to mutant ratio (2.1) is marked in red. NL-NanoLuc® tag, HT– HaloTag®Fusion tag, Ub – ubiquitin. See for details
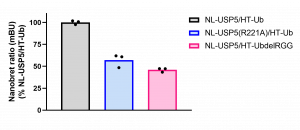
Fig.3. USP5 R221A mutation and ubiquitin RGG deletion decrease USP5 and ubiquitin interaction – NanoBRET assay. MCF7 were co-transfected NL- USP5 or USP5 R221A mutant and HT-Ub or UbdelRGG mutant. The signal was red 48 h post transfection. Experimental triplicates of mean corrected NanoBRET ratios (mBU) normalized to mBU of NL-USP5 and HT-ubiquitin are indicated. NL-NanoLuc® tag, HT– HaloTag®Fusion tag, Ub – ubiquitin.
