In my last blog post, I observed morphological changes that were indicative of an epithelial to mesenchymal transition (EMT) when NSD3short was overexpressed from a stably integrated transgene in the cancer cell line H1299. Importantly, NSD3 has been identified as amplified in a number of cancer types, including lung (Figure 1) (cBioPortal: Cerami et al., Cancer Discov. 2012 and Gao et al. Sci. Signal. 2013). Therefore, it is important to study how increased NSD3 levels may influence a cancer cell.
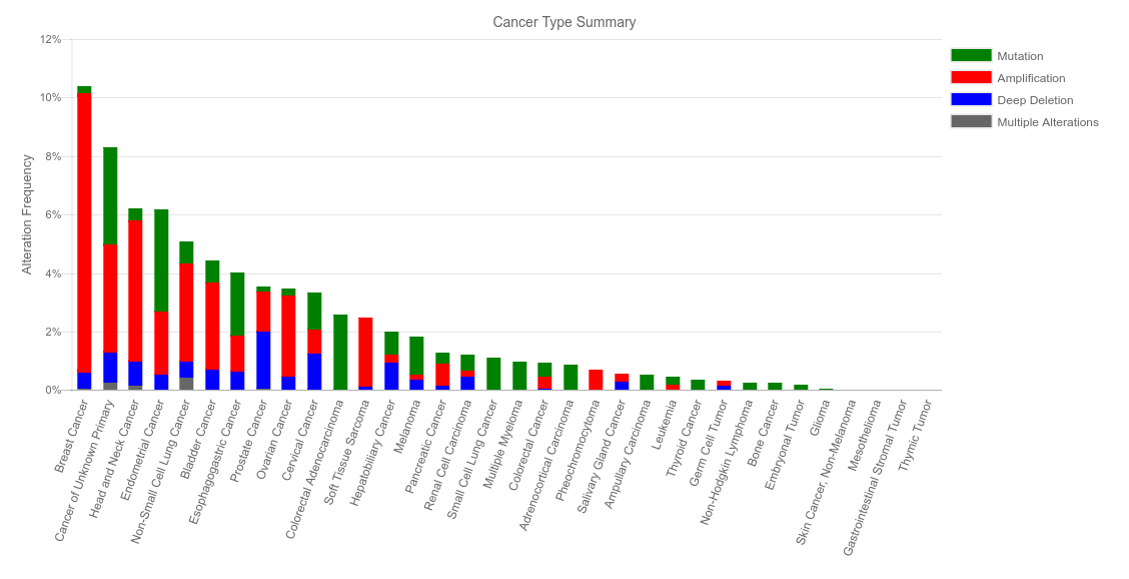
Figure 1. NSD3 Status Across Multiple Cancer Types (Data from cBioPortal)
To follow up on this putative phenotype, I have tested the same cell lines for E-cadherin expression and in wound healing assays (Figure 2 – full experimental details and results in the links). During epithelial to mesenchymal transitioning (EMT), transcription networks become activated and cytoskeletal rearrangements take place, promoting migratory capacity and invasiveness. Cadherins are a family of cell adhesion molecules that are important regulators of these events. In particular, E-cadherin, a marker for polarized epithelial cells, is downregulated during EMT. When we assay E-cadherin levels in the H1299 cell lines, we observe decreased expression with either wild-type or PWWP1 mutant (W284A) NSD3 transgene overexpression (Figure 2A). Next, to test if these cells display increased migratory capacity, I turned to a field standard, the wound healing assay. This involves making a scratch in a cell monolayer and measuring the extent to which cells migrate and divide into the cleared space over time. We observe that these cell lines appear to close wounds faster than the control (Empty Vector). Collectively, these results support the hypothesis that NSD3short is a potentiator of EMT, although PWWP1 function does not appear to be required. These results will be validated by repeating with independently transduced and puro-selected stable lines and in NSD3-knockdown conditions.
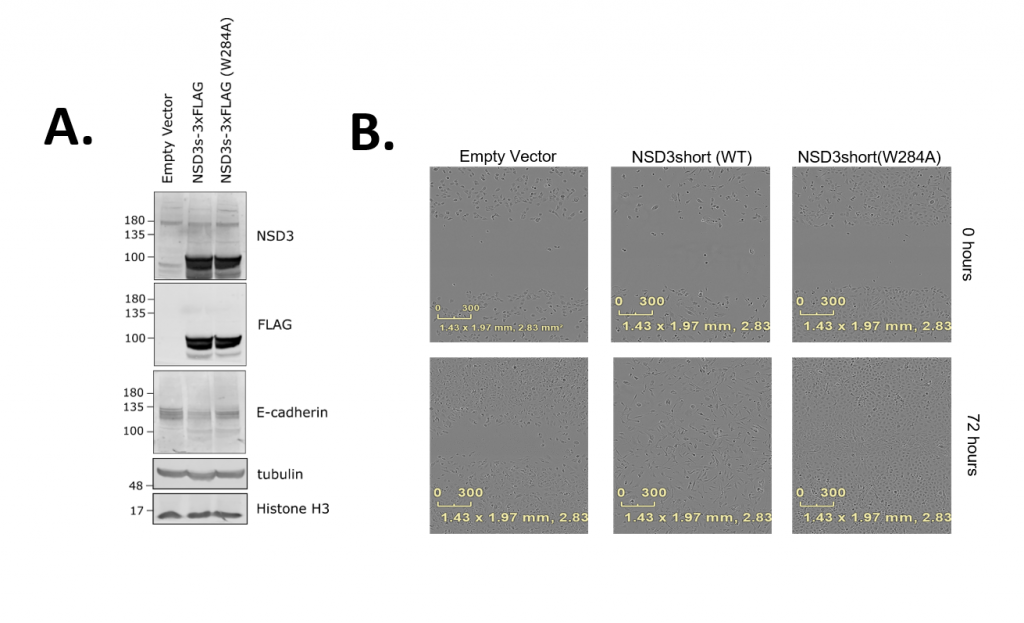
Figure 2. (A) Western blot for E-cadherin Expression (B) Representative Wells from a Wound Healing Assay at 0 and 72 hours

One Reply to “Following Up on NSD3’s Involvement in Epithelial to Mesenchymal Reprogramming”