Hi, my name is Ellie Williams and I’ve been working at the SGC since 2010 in the lab of Alex Bullock. I’m funded by FOP Friends to look at the development of new drugs that target ALK2 and to study the disease Fibrodysplasia Ossificans Progressiva (or FOP). In the last couple of years a new link has been spotted between the mutations found in FOP and DIPG (you can read more on Ros Adamson’s blog) with new possibilities of overlapping research helping both disease groups. The work I do will therefore be closely linked to that of Ros, Jong Fu and Liz although we’re all looking at different aspects of the larger problem.
FOP is a rare, progressive and debilitating condition caused by a single mutation in the ALK2 protein which is part of the BMP signalling pathway. Under normal circumstances it is responsible for regulating bone growth, however, in FOP patients a mutation in the protein causes dysregulation and over-activity of the pathway leading to inappropriate bone growth. This results in patients joints ‘locking up’ due to the growth of sheets and spurs of bone over joints that cannot be operated on as this results in even more bone growth. Bone growth appears to occur in ‘episodes’ with flare ups over a joint indicating the formation of new bone in the area.
In 97% of cases the mutation responsible is the change of an Arginine to a Histidine residue on a part of ALK2 that is both intracellular and directly adjacent to the kinase domain. This R206H mutation is the most common mutation but it is not the only one. Around a dozen other mutations have been identified in patients, all of them on the intracellular portion of the protein, and all in or very close to the kinase domain. Interestingly the R206H mutation has also been observed in 27% of cases of the rare childhood brain cancer DIPG – while the reason behind this is currently unclear, this opens up a new area of collaboration and research that might be beneficial for both patient groups.
So, back to the research – there are two key questions to consider when thinking about this project; the first is how can we stop it? Can we develop a potent and specific compound that will inhibit or damp down the aberrant signalling of mutant ALK2 and prevent disease; the second question is why do these mutations cause disease? What is the physical basis for the dysregulation and what influence does it have over the interaction of ALK2 with its standard binding partners and other proteins related pathways?
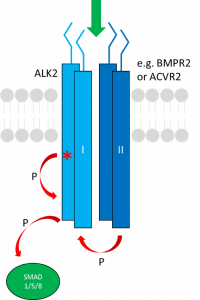
Outside activation (green arrow) results in ALK2 association with ACVR2 (or other type II receptor) and subsequent auto-phosphorylation and trans-phosphorylation by the type II receptor before activating SMAD1 via phosphorylation. The location of the FOP mutation is shown with the red *.
Both these aspects of research are important – finding potential drug molecules is key for any future treatment development, equally understanding the mechanism of the disease can help us spot new avenues for targeting future treatments or allow us to discover why certain things do or do not work.
To characterise the binding of new compounds with proteins I shall be using protein crystallography to study the co-crystallisation of ALK2 with various compounds. This will allow us to look in detail at how the compound binds within the binding pocket of ALK2. This can allow us to identify key contacts that should be maintained for binding, identify potential new contacts that can be developed in future compounds and identify regions that confer specificity between proteins. I will also be looking at how some compounds bind to the protein ALK5, which shows a very high structural similarity to ALK2, and for which it is important to develop selectivity against in order to minimise cross target effects for any future drug developed.
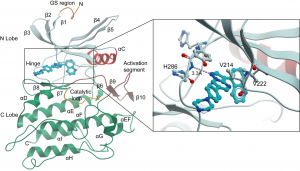
ALK2 co-crystallised with the ALK2 inhibitor LDN-212854 showing a key hydrogen bond between a nitrogen atom in the compound and the backbone nitrogen of Histidine 286.
In order to characterise the effect of the mutation on the protein I will be using a number of different techniques to look at the activity of ALK2 and it’s interaction with it’s binding partners. One of the main techniques I will be using is mass spectrometry to monitor the phosphorylation state of ALK2 under different conditions – looking at autophosphorylation, cross phosphorylation by different binding partners (such as BMP2 and ACVR2), phosphorylation of it’s target protein SMAD1 and how these are altered by the presence of mutations within ALK2.
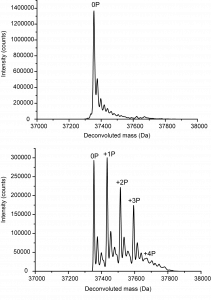
Mass spec experiment shows the unphosphorylated state of ALK2 (top panel) compared to the phosphorylated state (bottom panel) showing four phosphorylations on the protein.
This will be suplemented with western blot experiments to look at SMAD1 phosphorylation under different conditions, mutation experiments where phosphorylation sites in ALK2 will be altered to try and identify which are critical for activity, and a number of other complementary techniques as needed. This portion of the research also has the scope to look at other associated proteins, such as the inhibitor FKBP12 and the scaffolding protein Endofin for example, to see how they interact with ALK2 and how the FOP mutations influence their normal behaviour.
Over the coming months my experiments will be split between these two areas of investigation and report what I find here.
For more information you might find the following blog posts of interest:
https://openlabnotebooks.org/acvr1-the-link-between-fop-and-dipg/

Has the morpholinyl analogue of LDN-212854 been tested either in silico or in vitro? It seems like from the graphic you posted the hinge area would be tolerant to it?
We have tried the morpholine group as part of the LDN series both in our internal chemistry efforts and as part of a collaboration with ICR in London. There should be a paper published imminently containing the structure of one of these compounds along with some detailed discussion of the chemistry. The morpholinyl compounds were well tolerated, the group tends to point into the solvent channel and thus the hinge and back of pocket contacts are not disrupted. There is some indication that the morpholine group can form water mediated contacts with residues in the solvent channel, however this region in all the compounds we’ve studied tends to show a high flexibility regardless of group and thus doesn’t seem to provide significant extra specificity or potency.