In an effort to develop clinical compounds for Diffused Intrinsic Pontine Glioma (DIPG) treatment, new analogues of ACVR1/ALK2 inhibitors are continuously synthesised by Ontario Institute for Cancer Research (OICR) and Charles River Laboratories (CRL). I will provide prompt feedback of the cellular assay results to guide their design of new compounds.
HEK293 cells were nicely transfected, as indicated by GFP signal. I can proceed with the experiment.
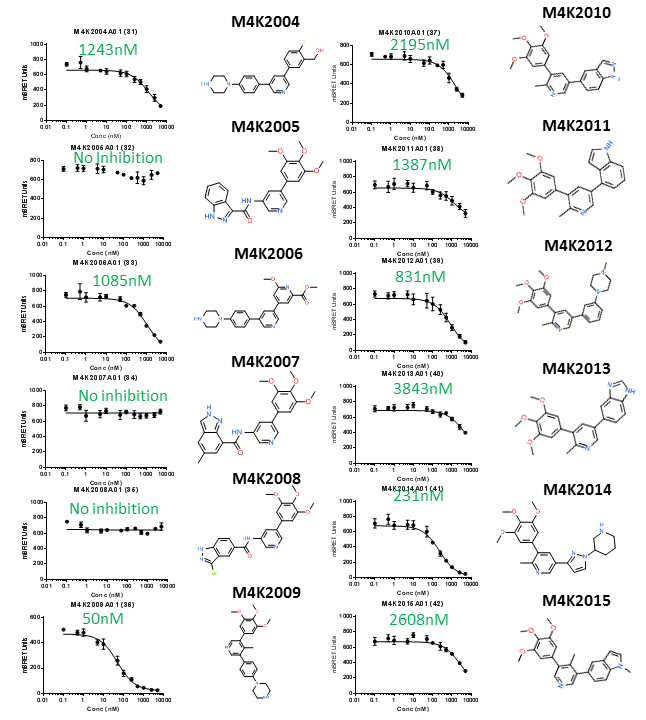
ALK2 NanoBRET IC50 curves and chemical structures of the first dozen of new ACVR1/ALK2 inhibitors. IC50 values estimated by GraphPad Prism are shown in green.
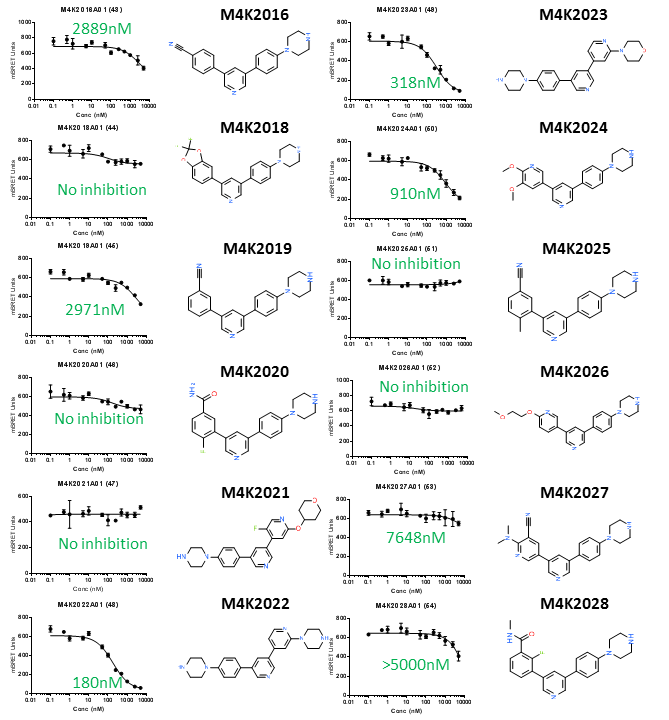
ALK2 NanoBRET IC50 curves and chemical structures of the second dozen of new ACVR1/ALK2 inhibitors. IC50 values estimated by GraphPad Prism are shown in green.
I determined the potency of 24 new ACVR1/ALK2 inhibitors synthesised by OICR. M4K2009 in particular is quite potent (larger values indicates lower potency). This compound differs from legacy compound M4K1055 by the position of its methyl group (CH3) in the middle of the molecule. Attempts to replace the tri-methoxy group (the three CH3-O groups bonded to the phenyl ring) were not successful. Since a robust nanoBRET tracer is not available for TGFBR1/ALK5, I will use dual luciferase promoter assay (orthologous assay) to determine the off-target activity of these compounds towards TGFBR1/ALK5.
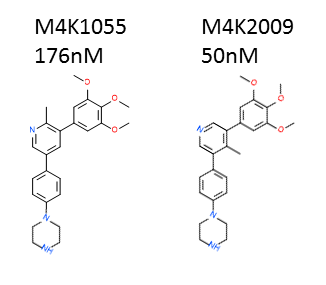
Comparing the chemical structures of M4K2009 to legacy compound M4K1055. ALK2 nanoBRET IC50 values of the compounds are also shown (lower values reflects increased potency).
The M4K monthly meeting where this result was presented has been recorded and posted on youtube.
For detailed experimental protocols, please refer to my Zenodo page.
