If you’ve been following along my open notebook journey over the last two years, you’ll be excited to hear I’ve published my work on the first antagonists of USP5 ZnF-UBD in a peer-reviewed journal. Check it out here!
In a previous post, I outlined my selection of the next set of analogues in my chemical series against USP5 ZnF-UBD to continue to understand the structure activity relationship and to improve potency. I used a displacement assay, which I described a few weeks ago to screen 20 commercial compounds that I had selected by virtual screening. The best displacing compounds were then tested using a surface plasmon resonance (SPR) assay. You can see details on Zenodo.
The displacement assay results are summarized in Figure 1. Compound displacement comparable to the control compound 21, a compound that has a KD of approximately 60 µM, were selected to be tested by SPR to determine binding affinities.
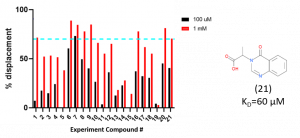
Figure 1. Summary of displacement screen
One of the most promising compounds, UBTR012574a has a KD of approximately 10 µM, a significant increase in potency from the parent molecules (Figure 2).
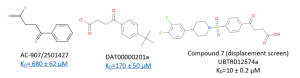
Figure 2. SAR of chemical series
In the next couple of weeks, I will try to solve the co-crystal structure of USP5 ZnF-UBD in complex with UBTR012574a to determine if the predicted binding pose is similar to the experimental binding pose. This will give us some insight into what chemical moieties of the compound are lending to the increased potency. I am also going back to Enamine’s REAL database and looking for compounds similar to UBTR012574a. I’ll be ordering some more analogues to get a better understanding of the structure activity relationship of the extended sulfonamide groups at the para position of the benzene ring. Stay tuned!
| Structure Image | Smiles | Mol. Weight |
|---|---|---|
| Cc1sc2ncn(CC(=O)[O-])c(=O)c2c1C | 237.3 | |
| CC(=O)N1CCN(C(=O)CCC(=O)[O-])CC1 | 227.2 | |
| O=C(O)Cn1ccc2ccccc2c1=O | 203.2 | |
| CSc1nc(C)c(CCC(=O)[O-])c(=O)[nH]1 | 227.3 | |
| O=C(O)CN1Cc2ccccc2C1=O | 191.2 |
