Background
WDR5 belongs to the WD-repeat protein family and is an important component of various complexes, including SET1/MLL, NuRD, and NSL complex. It interacts with several binding partners via the “WDR5-interacting” (Win) site (e.g. symmetrically dimethylated histone H3R2, MLL1-4, SET1-2) or “WDR5-binding motif” (WBM) site (e.g. c,l,n-MYC, RBBP5). WDR5 emerged as a prime target for anti-cancer therapy due to its important roles in gene transcription, proliferation, and self-renewal (PIMID 29385767).
Assay validation
Assay measuring the interaction of WDR5 with histone H3 was developed using NanoBRET technology – a proximity-based assay that can detect protein interactions by measuring energy transfer from a bioluminescent protein donor to a fluorescent protein acceptor. We tested different donor (N- or C-terminally NanoLuc® tagged WDR5) to acceptor (C-terminally HaloTag®Fusion-tagged histone H3.3) ratios in two different cell lines (U2Os and HEK293T). The assay was validated with WDR5 Win site antagonist – OICR9429 (PIMID:2695870). The best NanoBRET ratio and the greatest compound effect was obtained in U2Os cells transfected with 0.01 ug of C-terminally HaloTag®Fusion-tagged histone H3.3 and 0.001 µg of C- terminally NanoLuc® -tagged-WDR5 per 96-well (Fig.1).
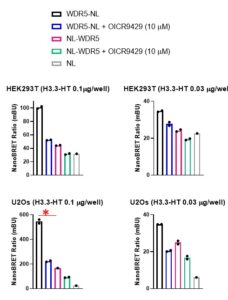
Fig.1. OICR9429 decreases WDR5 interaction with histone H3.3 – NanoBRET assay. U2Os or HEK293T cells were co-transfected with 0.001 µg of N- or C-terminally NL-tagged WDR5 and different amounts of C-terminally HT-tagged histone H3.3, as indicated (per 96/well) and treated with OICR9429 for 4 h. * – best assay condition, NL-NanoLuc® tag, HT- HaloTag®Fusion tag
Experimental details can be found on Zenodo.
