Kun Qian, Ranjita Betarbet, Rachel Commander, Erik Johnson,
Opher Gileadi, Haian Fu, and Allan Levey
https://doi.org/10.5281/zenodo.4900586
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, characterized by two pathological hallmarks – extracellular amyloid plaques that are made up of amyloid-peptide and intracellular neurofibrillary tangles that are comprised of hyperphosphorylated tau. AD neuropathology, however, begins decades before symptomatic cognitive decline. To understand early changes associated with AD pathogenesis and to identify new targets for early diagnosis and therapeutics, our group has initiated a thorough examination of the human AD brain proteome. As published previously (1) and more recently (2), weighted co-expression network analysis of the AD brain proteome revealed a module that was strongly correlated with higher neuropathological burden and worse cognitive outcomes, as well as highly enriched in microglial and astrocytic proteins and contained proteins involved in response to inflammation. One of the key proteins within this module, CD44 (cluster of differentiation 44), has been identified as a proinflammatory protein. The CD44 gene has also been specifically linked to AD in a genome-wide association study, where CD44 was identified as one of several genes that are associated with modification of the age of onset of AD in PSEN1 mutation carriers (3). In this report we have investigated the association of CD44 and its isoforms to AD pathology.
CD44 is an inflammation-related gene encoding a widely distributed family of alternatively spliced cell surface glycoproteins (4). CD44 functions as a receptor for hyaluronic acid (HA) and osteopontin, and has been implicated together with its ligand HA in several inflammatory diseases, in inflammation-linked neuronal injuries and in cancer (4). Numerous CD44 isoforms are generated due to alternative RNA splicing which involves at least 10 exons (5). In addition, variations in glycosylation contribute to structural and functional diversity of CD44. Standard CD44 (CD44s) lacks all 10 variable exons while variant CD44 isoforms may contain one or more alternatively spliced exons in between standard exons (5). The distribution of the CD44 isoforms in the normal (5) and AD brain have been reported previously (4). However, the specificity of the isoform-specific antibodies was not verified in these previous studies. In this investigation we have first confirmed the specificity of the CD44 isoform-specific antibodies and then used the validated antibodies to examine the distribution of CD44 isoforms in normal and AD brains.
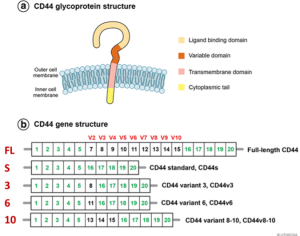
Figure 1. Structure of the CD44 variants from Chen et al. (Reference 6). (A) Illustration of the domain structure of CD44 glycoprotein. (B) Schematic of CD44 variants. Exons shown in green are always expressed as a standard form of CD44 (CD44s), and up to nine exon variants can be inserted by alternative splicing. Full-length CD44, CD44s, CD44v3, CD44v6, and CD44v8-10 are shown. Figure from Chen et al. (6).
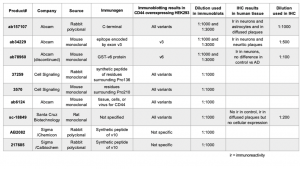
To confirm the specificity of the commercial CD44 antibodies, we obtained plasmid constructs of the five CD44 isoforms (Figure 1) fused with a C terminal V5 tag. These plasmids were transfected individually into HEK293T cells and the cells overexpressing the corresponding CD44 isoform were subjected to immunoblotting detection using the CD44-specific antibodies obtained from several commercial sources. The immunoblotting results are shown in Figure 2 and the antibody information can be found in Table 1. Pan-CD44 antibodies are generated from protein sequences shared by all CD44 isoforms. They recognize all five isoforms in the immunoblots (Figure 2A). Antibody sc-18849 is described as CD44s specific, however, the exons for the CD44s isoform are shared with all CD44 isoforms. Therefore, sc-18849 should not be specific to CD44s and is included as a pan-CD44 antibody. The CD44 v3 specific antibody is generated from an epitope encoded by exon 9 (Figure 1), which identifies the full length CD44 and CD44 v3, but not others (Figure 2B). Similarly, the epitope for CD44 v6 antibody is encoded from exon 11 (Figure 1), which identifies the full length CD44 and CD44 v6, but not others (Figure 2C). The CD44 v6 antibody shows nonspecific signal at a 1:1000 dilution, but the non-specificity is significantly reduced when using a 1:3000 dilution. All the three CD44 v10 antibodies are nonspecific (Figure 1D). While ab6124 can identify all of the CD44 isoforms, the 217685 and AB2082 only show non-specific background signal. These results are summarized in Table 1.
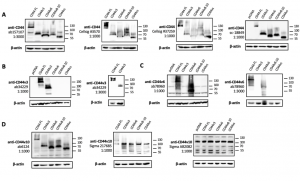 Figure 2. Validation of CD44 antibodies in HEK293T cells overexpressing CD44 variants. (A) blots for pan-CD44 antibodies, (B) blots for a CD44 v3 specific antibody at 1:1000 and 1:3000 dilution, (C) blots for a CD44 v6 specific antibody at 1:1000 and 1:3000 dilution, (D) blots for CD44 v10 antibodies. The primary antibody used in each blot is identified by the product number and followed by the dilution factor for incubation. Samples are labeled with the plasmid for overexpression: CD44 FL – full-length CD44; CD44v3 – CD44 variant 3; CD44v6 – CD44 variant 6; CD44v10 – CD44 variant 10; CD44s – CD44 standard. β-actin is used as loading control.
Figure 2. Validation of CD44 antibodies in HEK293T cells overexpressing CD44 variants. (A) blots for pan-CD44 antibodies, (B) blots for a CD44 v3 specific antibody at 1:1000 and 1:3000 dilution, (C) blots for a CD44 v6 specific antibody at 1:1000 and 1:3000 dilution, (D) blots for CD44 v10 antibodies. The primary antibody used in each blot is identified by the product number and followed by the dilution factor for incubation. Samples are labeled with the plasmid for overexpression: CD44 FL – full-length CD44; CD44v3 – CD44 variant 3; CD44v6 – CD44 variant 6; CD44v10 – CD44 variant 10; CD44s – CD44 standard. β-actin is used as loading control.
Based on the immunoblotting results, pan-CD44 (ab157107), CD44v3, and CD44v6 antibodies were selected to further investigate the CD44 isoform expression levels in normal and AD brains (Figure 3-7). The pan-CD44 antibody showed immunoreactivity in neurons and astrocytes in control brains (Figure 3) and in diffused plaque-like structures and neuritic profiles in AD brains (Figure 4). The CD44 v3 antibody showed immunoreactivity, primarily in neurons in control brains (Figure 5 A-C) and in neuritic plaque-like structures and dystrophic neurites in AD brains (Figure 5 D-F & 6). The CD44 v6 antibody showed immunoreactivity in neurons, however, no differences were observed between the control and AD samples (data not shown). The CD44s antibody, which is nonspecific and recognizes all CD44 isoforms in the immunoblotting results, did not show reactivity in the control brain tissue but showed immunoreactivity in diffused plaques in AD brains, without specific cell-type expression (Figure 7).
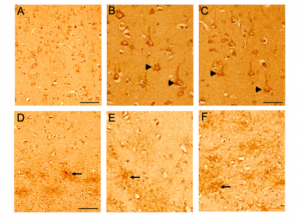
Figure 3. CD44 expression in control brains using pan CD44 antibody (ab157107, antibody specific to the C-terminal epitope 692-742aa). Paraffin embedded brain sections from the frontal cortex were processed for immunocytochemistry. Representative images demonstrating CD44-Immunoreactivity (ir) in control (Figure 3) and AD (Figure 4) brain sections. Immunoreactivity (ir) for CD44, using the CD44 AB 157107 antibody, is detected in both neuronal (Figure 3A, B, C) and astrocytic (Figure 3D, E, F) cell types in the control brain sections throughout the frontal cortex. CD44 ir can be observed in neuronal (arrow head) cell body and processes and also in astrocytes (arrow) and astrocytic processes in the white matter region.
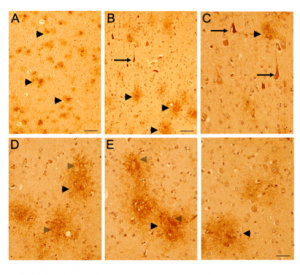 Figure 4. CD44 expression in AD brains using pan CD44 antibody (ab157107, antibody specific to the C-terminal epitope 692-742aa). Paraffin embedded brain sections from the frontal cortex were processed for immunocytochemistry. Representative images demonstrating CD44-Immunoreactivity (ir) in control (Figure 3) and AD (Figure 4) brain sections. In AD brain sections (Figure 4 A-F), CD44-ir is detected in diffused plaque-like structures (Figure 4, black arrowhead) throughout the cortical layers and in the white matter regions. At a higher magnification, CD44-ir can be detected in astrocytic soma (Figure 4, grey arrow head) and processes surrounding the diffused plaques. CD44-ir neuritic structures (Figure 4 B, C, arrow) can also be observed throughout the cortex. Scale bars – 3A, 4A – 200µm, All other figs – 100µm.
Figure 4. CD44 expression in AD brains using pan CD44 antibody (ab157107, antibody specific to the C-terminal epitope 692-742aa). Paraffin embedded brain sections from the frontal cortex were processed for immunocytochemistry. Representative images demonstrating CD44-Immunoreactivity (ir) in control (Figure 3) and AD (Figure 4) brain sections. In AD brain sections (Figure 4 A-F), CD44-ir is detected in diffused plaque-like structures (Figure 4, black arrowhead) throughout the cortical layers and in the white matter regions. At a higher magnification, CD44-ir can be detected in astrocytic soma (Figure 4, grey arrow head) and processes surrounding the diffused plaques. CD44-ir neuritic structures (Figure 4 B, C, arrow) can also be observed throughout the cortex. Scale bars – 3A, 4A – 200µm, All other figs – 100µm.
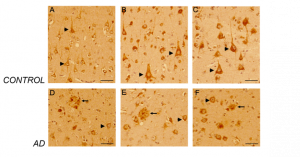 Figure 5. CD44 V3–isoform specific expression in control and AD brains. Representative images demonstrating immunoreactivity (ir) for CD44 V3 isoform, using the CD44 V3-specific AB 34229 antibody. CD44 V3–isoform specific ir was detected primarily in neuronal cell type throughout the cortex (Figure 5 A-F, arrowhead). CD44 V3 ir was detected in the neuronal soma and processes (Figure 5 A-F). Note the lack of CD44 V3 ir in other brain cell types. In AD brain sections (Figure 5, 6), CD44 V3 ir was detected in neuritic plaques (Figure 5 D-F; Figure 6, arrows) and in some dysmorphic/abnormal neurites throughout the cortical layers and in some tangle-like structures (Figure 6 C, D, E, arrowhead). Scale bars – Figure 5A, D – 200µm, All others – 100µm.
Figure 5. CD44 V3–isoform specific expression in control and AD brains. Representative images demonstrating immunoreactivity (ir) for CD44 V3 isoform, using the CD44 V3-specific AB 34229 antibody. CD44 V3–isoform specific ir was detected primarily in neuronal cell type throughout the cortex (Figure 5 A-F, arrowhead). CD44 V3 ir was detected in the neuronal soma and processes (Figure 5 A-F). Note the lack of CD44 V3 ir in other brain cell types. In AD brain sections (Figure 5, 6), CD44 V3 ir was detected in neuritic plaques (Figure 5 D-F; Figure 6, arrows) and in some dysmorphic/abnormal neurites throughout the cortical layers and in some tangle-like structures (Figure 6 C, D, E, arrowhead). Scale bars – Figure 5A, D – 200µm, All others – 100µm.
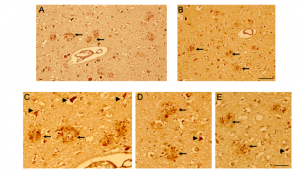 Figure 6. CD44 V3–isoform specific expression in AD brains. Representative images demonstrating immunoreactivity (ir) for CD44 V3 isoform, using the CD44 V3-specific AB 34229 antibody. In AD brain sections (Figure 5, 6), CD44 V3 ir was detected in neuritic plaques (Figure 5 D-F; Figure 6, arrow) and in some dysmorphic/abnormal neurites throughout the cortical layers and in some tangle-like structures (Figure 6 C, D, E, arrowhead). Scale bars – 100µm.
Figure 6. CD44 V3–isoform specific expression in AD brains. Representative images demonstrating immunoreactivity (ir) for CD44 V3 isoform, using the CD44 V3-specific AB 34229 antibody. In AD brain sections (Figure 5, 6), CD44 V3 ir was detected in neuritic plaques (Figure 5 D-F; Figure 6, arrow) and in some dysmorphic/abnormal neurites throughout the cortical layers and in some tangle-like structures (Figure 6 C, D, E, arrowhead). Scale bars – 100µm.
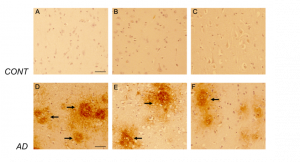 Figure 7. CD44s-isoform specific expression in control and AD brains. Representative images demonstrating immunoreactivity (ir) for CD44s isoform, using the CD44s-specific sc18849 antibody. Note that the CD44s-ir could not be detected in the control brain sections (Figure 7 A-C) using this antibody. In AD brain sections (Figure 7 D-F), CD44s-ir could be detected in diffused-plaque-like structures (arrow) throughout the frontal cortex. The ir was not associated with any brain specific cell types. Please note that sc18849 antibody is not specific for the standard CD44 isoform (CD44s, Figure 2). Scale bars – 100µm.
Figure 7. CD44s-isoform specific expression in control and AD brains. Representative images demonstrating immunoreactivity (ir) for CD44s isoform, using the CD44s-specific sc18849 antibody. Note that the CD44s-ir could not be detected in the control brain sections (Figure 7 A-C) using this antibody. In AD brain sections (Figure 7 D-F), CD44s-ir could be detected in diffused-plaque-like structures (arrow) throughout the frontal cortex. The ir was not associated with any brain specific cell types. Please note that sc18849 antibody is not specific for the standard CD44 isoform (CD44s, Figure 2). Scale bars – 100µm.
In brief, we confirmed the specificity of commercially available CD44 antibodies by immunoblotting transfected cell samples overexpressing specific CD44 variants. We then applied the validated antibodies to examine the distribution of CD44 variant expression levels in normal and AD brain tissues using immunohistochemistry. The experimental conditions and results of this study are summarized in Table 1.
Table 1. Commercial antibodies used in immunoblotting and immunohistochemistry.
Methods
Plasmid constructs
Sequences of CD44 variants with a C terminal V5 tag were cloned into the FUW backbone between XbaI and EcoRI restriction sites. The sequences of the CD44 variants used in this study are shown here.
Cell Culture and Immunoblotting
HEK293T cells (ATCC® CRL-3216™) cultured in DMEM with 10% FBS and 100 U/mL penicillin and streptomycin were plated at 5 x 105 cells per well in 6-well plates. On the next day when the cell confluency reached 70%, CD44 variant plasmid or pcDNA control plasmid was transfected at 2 µg per well with transfection reagent polyethylenimine at 1:3 ratio (6 µL of reagent per well). Cells were harvested 45 hours post-transfection, then lysed and boiled with 200 µL of 2X Laemmli Sample Buffer (BIORAD Cat#1610737). The samples were used for SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% milk/TBST buffer, membranes were incubated with primary antibodies at 1:1000 or 1:3000 dilution at 4 oC overnight, then incubated with corresponding HRP-conjugated secondary antibodies at 1:5000 or 1:10,000 dilution for 1 hr at room temperature. Membranes were exposed with SuperSignal West Pico PLUS chemiluminescence substrate (Thermo Scientific, REF 34580) and images were taken by BIORAD ChemiDoc Touched Imaging System.
Immunohistochemistry
Cryopreserved, pathology-confirmed AD (N = 10, 5males, 5 females, average age at death = 72.7) and non-disease control (N = 10, 5 males, 5 females, average age at death = 61.4), formalin fixed, paraffin embedded, frontal cortex tissue blocks were obtained from the Emory Neuropathology/ Histochemistry Core. 4-5um paraffin sections were deparaffinized and immuno-stained with CD44 isoform-specific antibodies (listed in Table 1) on a Thermo Fisher autostainer. Immunohistochemistry was carried out using a Vector ImmPRESS kit per manufacturer instructions. Briefly, sections were pretreated by exposure to hot citrate buffer, then blocked with Bloxall (10 minutes, room temperature) followed by 2.5% normal serum (20 minutes, room temperature). Sections were subsequently incubated with primary antibody (60 minutes, room temperature), amplifier secondary antibody (30 minutes, room temperature) and ImmPRESS HRP anti-secondary polymer reagent (30 minutes, room temperature), then developed with diaminobenzidine (DAB). For controls primary antibodies were were omitted. Immuno-stained sections were mounted, and cover-slipped. Images were captured using an Olympus bright-field and camera (OlympusBX51). For final output, images were processed using Adobe Photoshop software.
Acknowledgements
This work was supported by the Emory Center for Neurodegenerative Disease Neuropathology and Viral Vector Cores, Emory Integrated Genomics Core, and the NIH National Institutes on Aging TREAT-AD Center for Alzheimer’s Disease grant U54AG065187.
References
- Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, Yin L, Deng Q, Nguyen T, Hales CM, Wingo T, Glass J, Gearing M, Thambisetty M, Troncoso JC, Geschwind DH, Lah JJ, Levey AI. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst. 2017 Jan 25;4(1):60-72.e4. doi: 10.1016/j.cels.2016.11.006.
- Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020 May;26(5):769-780. doi: 10.1038/s41591-020-0815-6.
- Vélez JI, Chandrasekharappa SC, Henao E, Martinez AF, Harper U, Jones M, Solomon BD, Lopez L, Garcia G, Aguirre-Acevedo DC, Acosta-Baena N, Correa JC, Lopera-Gómez CM, Jaramillo-Elorza MC, Rivera D, Kosik KS, Schork NJ, Swanson JM, Lopera F, Arcos-Burgos M. Pooling/bootstrap-based GWAS (pbGWAS) identifies new loci modifying the age of onset in PSEN1 p.Glu280Ala Alzheimer’s disease. Mol Psychiatry. 2013 May;18(5):568-75. doi: 10.1038/mp.2012.81.
- Pinner E, Gruper Y, Ben Zimra M, Kristt D, Laudon M, Naor D, Zisapel N. CD44 Splice Variants as Potential Players in Alzheimer’s Disease Pathology. J Alzheimers Dis. 2017;58(4):1137-1149. doi: 10.3233/JAD-161245.
- Kaaijk P, Pals ST, Morsink F, Bosch DA, Troost D. Differential expression of CD44 splice variants in the normal human central nervous system. J Neuroimmunol. 1997 Mar;73(1-2):70-6. doi: 10.1016/s0165-5728(96)00167-1.
- Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018 May 10;11(1):64. doi: 10.1186/s13045-018-0605-5.
