ALK2 and ALK5 are Type I receptors on the cell surface that can be activated by specific ligands (illustrated in the diagram below). Once activated by ligand, ALK2 or ALK5 interact with Type II receptor to be phosphorylated (phospho-group chemically attached to the protein). Subsequently, ALK2 phorphorylates SMAD1/5/8 while ALK5 phorphorylates SMAD2. Phosphorylated SMADs then translocate to the cell nucleus to regulate gene transcription. ALK2 is mainly activated by BMP4, BMP6, BMP7 and BMP9 while ALK5 is mainly activated by TGFβ and BMP2.
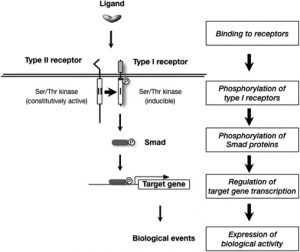
Scheme of ALK2/ALK5 (both are Type I receptors) activation by ligands
Considerable amount of ligands will be consumed in the dual luciferase assay (DLA) which will be used for the screening of ALK2 inhibitors and off-target inhibition of ALK5 routinely. Ligands are costly to procure. In order to minimise wastage, it is important to determine the least amount of ligands needed to achieve optimal stimulation of ALK2 and ALK5.
In these experiments, the activation of ALK2 and ALK5 by different concentrations of ligands in MDA-MB-231 reporter cells was analysed in Western Blot and DLA. For full experimental details, please visit Zenodo.

Activation of ALK2 (BRE) and ALK5 (CAGA) by different ligands as quantified in DLA
