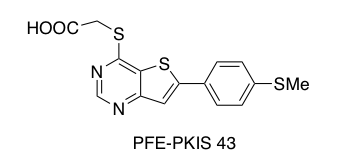
Towards the SGC goal of creating a kinase chemogenomic set (KCGS) we have been receiving and profiling kinase inhibitors donated by pharmaceutical companies. One such compound is PFE-PKIS 43 (Scheme 1a). We received this compound from Pfizer and it was screened against 403 kinases at DiscoverX. This compound, originally synthesized and published as part of a TPL2 kinase inhibitor program, was found to have a Kd of 220 nM and 3.8 nM for DRAK1 and DRAK2 respectively. This compound also demonstrated very little activity on other kinases tested however the DiscoverX panel does not include TPL2. DRAK2(STK17B) is a serine/threonine kinase associated with autoimmune diseases.
Our initial efforts are focused on the synthesis of several thieno[3,2]pyrimidines to generate an understanding of how structural modifications modulate DRAK1 and DRAK2 activity. Synthesis of these thienopyrimidines such as 5 is shown in scheme 1b. The synthetic route (Scheme 1b) begins with the deprotonation/iodination of 1, nucleophilic aromatic substitution on substrate 2, saponification of 3 followed by Suzuki coupling of 4 with different arylboronic acids. (Procedure adapted from Ni, Y. and co-workers. Bioorg. Med. Chem. Lett. 2011, 21, 5952-5956.)
a.
b.
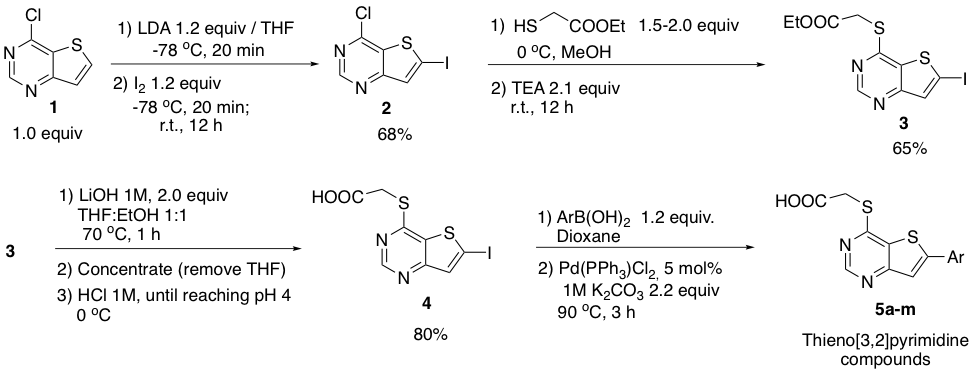
Scheme 1. (a) Structure of PFE-PKIS 43 donated by Pfizer (b) Synthesis of thienopyrimidine derivatives.
