The original compound donated by Pfizer PFE-PKIS 43 (thienopyrimidine scaffold), showed activity against 3 kinases greater than 70% inhibition in the KinomeSCAN at 1 µM (STK17B/DRAK2, AURKB and SRPK2).
In the series of compounds recently synthesized as thienopyrimidine derivatives we seek selective and potent inhibitors for STK17B/DRAK2 only.
To further identify off-target activity of these compounds, NanoBRET assays were implemented in our laboratory to test their potency (IC50) with AURKB and STK17A/DRAK1 in cells. These cellular assays were performed by Carrow Wells and Julie Pickett.
A set of STK17B/DRAK2 inhibitors were tested against AURKB, results are shown below.
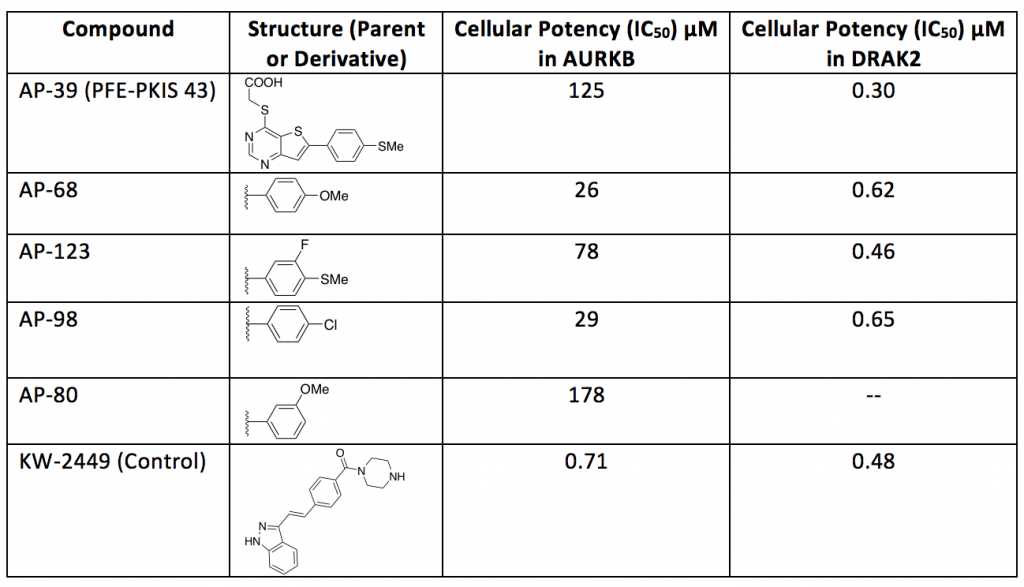
Table 1. Potency of selected compounds against AURKB and DRAK2
Table 1 shows good news in terms of selectivity. All thienopyrimidine compounds resulted inactive towards AURKB while maintaining good cellular potency with DRAK2. AP-39 shown an IC50 = 19 µM for DRAK1 in the nanoBRET. Compound KW-2449 was used as a control.
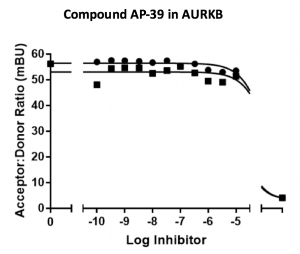
Figure 1. Activity of compound AP-39 with kinase AURKB, nanoBRET assay.

What is known about the Km for ATP on DRAK2 and AURKB? Your results suggest that AURKB has a much higher affinity than DRAK2 for ATP in cells.