After preparation of a set of GW807982X analogs, the next step was to get screening data against CLK1/2/3 at concentration of 1 uM at Luceome. For compounds that showed an inhibition of >75% on target they get a full dose response curve to generated IC50 values. To date, a set of 60 compounds have been synthesized (chemistry can be checked here: https://openlabnotebooks.org/synthetic-highlights-for-clk-inhibitors-and-shiny-science/), and some of the interesting results are shown below.
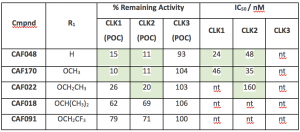
CAF022, R1 = Ethoxy, showed potent activity against CLK2 with an IC50 of 160 nM, and exploring the R1 substitution provided evidence that the bigger it gets, the worse the activity on CLK1/2. When going from an ethoxy (CAF022) to an isopropoxy (CAF018) substitution, the inhibition is compromised on CLK1 and 2. Whereas, introduction of a smaller methoxy group (CAF170) or just hydrogen (CAF048) the inhibition is greater (CLK 2 IC50 35 nM and 48 nM, respectively). With these results, we decided to keep making analogs with R1= H/Ethoxy.
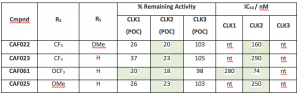
Different anilines were introduced, with different electronics and sterics. Some interesting results are shown below and preliminary hypothesis can be extracted. When compared to the parent (CAF022, R3= CF3, R4= OMe), either substituent on its own (CAF023, R3= CF3, R4= H and CAF025, R3= OMe, R4= H) showed no further improvement (CLK2 IC50 = 290 and 250 nM, respectively). Interestingly introduction of a m-OCF3 (CAF061) provided a clear enhancement with a CLK2 IC50 = 74 nM.
Furthermore, some compounds were tested to establish cell target engagement using a NanoBRET binding assay – IC50 curves were generated. Parent compound CAF022 provided an IC50 of 32 nM in cells whereas, CAF061 improved potency with an IC50 of 24 nM in cells. With potent compounds in hand, the next step is to check selectivity by Kinome Scan at DiscoverX. In order to be a potential chemical probe, compounds have to meet the SGC criteria, in where selectivity must be >30-fold across the kinome. CAF061 provided a fairly clean kinome tree, 5 kinases (including CLK1/2/4 and HIPK1/2) showed an inhibition greater than 90%, followed by 5 extra kinases with inhibition between 90-75%, so overall this is a potent and selective compound.
Now the plan is to prepare a few extra analogs to make sure we explored all the possible substitution patterns before we choose one of these analogs as a chemical probe. Feel free to suggest further ideas and if you have any questions or comments or want more detail on anything, please let me know and feel free to contact me.

Good stuff but please add SMILES strings to specify the explicit structures