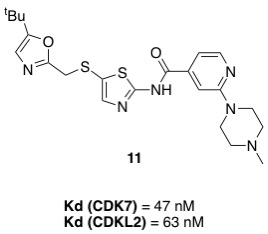
Following previous post, https://openlabnotebooks.org/sns-032-analogues-update/, compound 11 was chosen to further investigate the structure activity relationships (SAR) of the oxazole ring system with the hope to enhance potency and improve selectivity towards each respective target.
The criteria to pick the building blocks to further study the SAR is based on electronics, size and shape. I have synthesized twenty additional compounds as shown below. Introduction of phenyl and substituted phenyl rings have been prepared (1–7), with both electron donating and electron withdrawing substituents, with the purpose to observe the influence of ring substitution and activity. A more basic pyrimidine ring (8), was also prepared to probe potential hydrogen bond interactions with the kinases. Para-substituted amide functionalities on the phenyl ring were also introduced, including different sizes and basicity properties (9–12). We were also interested to prepare aliphatic side chains with different functionalities (14–20) with basic, acidic and bulkier groups to further elucidate the requirements of this pocket.
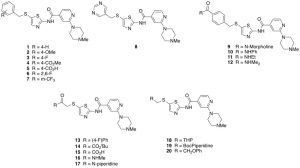
Overall, the building blocks I have introduced contain different functionalities, and provide for relatively big changes on the scaffold. This broad-brush approach allows for a deeper investigation of the chemotype and increases the chances for interesting results from the screening. In the coming weeks, I will send compounds 1–20 to DiscoverX to get Kd’s on the understudied kinases we are interested, I wil share this as it arrives. Do feel free to suggest further ideas and if you have any questions or comments or want more detail on anything, please let me know.
