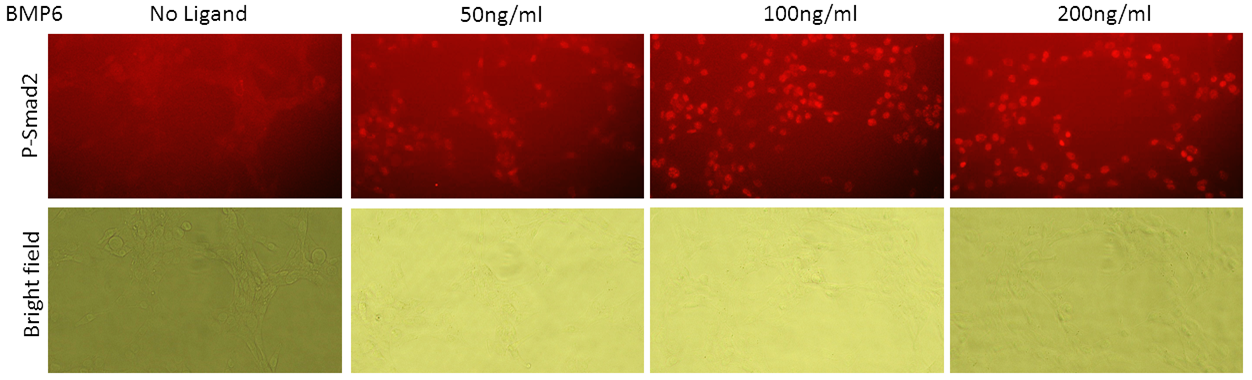
Kinase activity of ALK2 and ALK5 in the cells can be quantified using specific antibodies that bind to target proteins once they are phosphorylated. However, conventional detection in Western blot requires large amount of samples and processing time (proteins are extracted from a large number of cells, linearized, separated in electric field and blotted onto a membrane to be recognized by antibodies). The screening process can be made more high-throughput if the antibodies can be used to detect phosphorylated substrate directly in the cells. When fixed and stained in the cells, proteins remained folded. Epitopes that are recognized by antibodies might remain hidden or modified by formaldehyde crosslink.
According to the experimental results, anti-phospho-SMAD1/5 antibody can still recognize its epitope in formaldehyde-fixed cells. Unfortunately, anti-phospho-SMAD2 antibody did not stain specifically in fixed cells. For more experimental details, please refer to Zenodo.