In a previous post, I showed the expansion of UBTR012574 chemical series from commercial sources- Enamine’s REAL database and Scifinder to shed some insight into the structural activity relationship (SAR) (Figure 1).
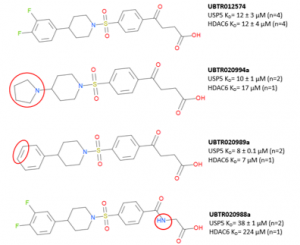
Figure 1. UBTR012574 chemical series
UBTR020988a which has an amide resulted in weaker potency for USP5 but is 6-fold selective for USP5 over HDAC6. Keeping the SAR in mind, we hope to improve potency and retain selectivity for USP5 ZnF-UBD. After exhausting commercial sources of compounds against USP5 ZnF-UBD, we started a collaboration with chemists at the Ontario Institute for Cancer Research (OICR) to continue to expand the UBTR012574 chemical series.
I docked 124 analogues of UBTR012574 generated by Dr. Carlos Zepeda and Hector Gonzaled-Alvarez at OICR based on ease of synthesis and available starting materials to USP5 ZnF-UBD to help prioritize synthesis. All docked analogues are amides, to retain selectivity based on SAR (UBTR20988) as well as easier chemistry. You can find details on Zenodo.
Of the docked analogues, there were two docking poses where (1) an aromatic ring makes pi-stacking interactions with Y223 (Figure 2- cyan) or (2) an ammonium group forms an H-bond with D264 (Figure 2-green).
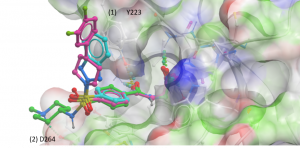
Figure 2. USP5 ZnF-UBD co-crystal structure with UBTR012574 (magenta) and docked poses of analogues (cyan & green)
This data was shared with chemists to be used to prioritize the synthesis of the first subset of analogues. The compounds with the best docking scores and poses will be synthesized in the first instance. Starting materials have been ordered; however, synthesis has been put on hold for COVID-19 related lab closures. Once labs re-open, we hope to get started on synthesizing and testing some of these compounds. Stay tuned!
