In searching for novel drugs that will treat future viral pandemics, the AViDD program has prioritized viral proteases, helicases, and RNA-dependant RNA polymerases (RdRps) as key targets in the fight to control viral replication.
At the time of writing, several drugs are either in the clinic or on the market for SARS-CoV-2 proteases and RdRps, while little is known about inhibitors of viral helicases. However, many drugs and inhibitors have been developed for human helicases and making analogs of these compounds may be a good starting point for discovering helicase inhibitors for viral proteins. While surveying the landscape of human helicase inhibitors, a manuscript describing potent inhibitors of Brr2 highlighted (1) (Fig. 1) as a 79 nM inhibitor (Iwatani-Yoshihara et al., 2017).
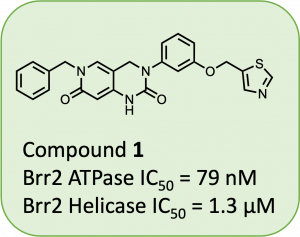
Figure 1: Reported human Brr2 helicase inhibitor
In an effort to develop inhibitors of viral helicases, we designed a small (10 x 10) library around this template using diverse building blocks around the heterocyclic core (Fig. 2).
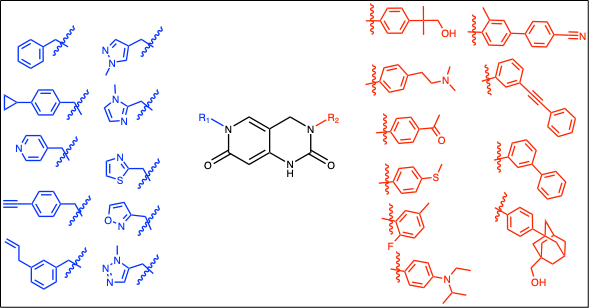
Figure 2: Brr2 library matrix with the variable R1 and R2
The very general synthetic scheme for this series of molecules is shown in scheme 1 with additional modification from the reported protocol by Iwatani-Yoshihara et al., 2017.
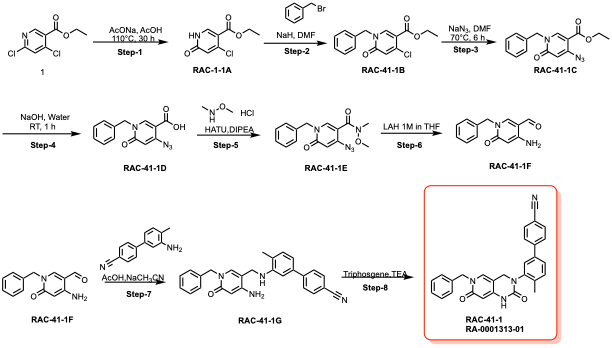
Scheme 1: General synthetic scheme of the Brr2 series compounds (Chemistry courtesy: CRO, Piramal Pharma Solution)
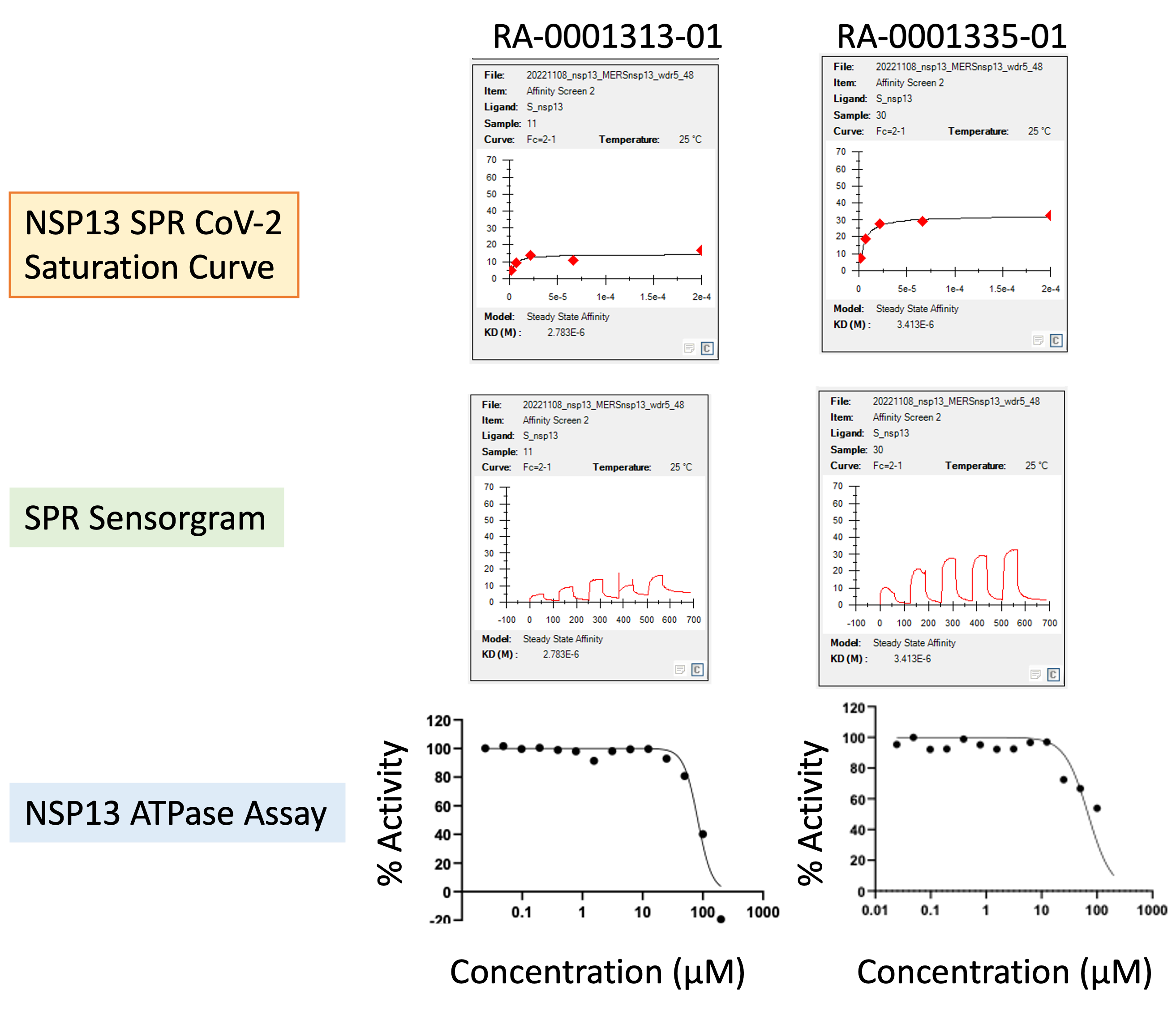
At the time of writing (Jan 2023), about 70% of projected compounds (> 150 analogs) have been synthesized and tested, giving a couple of compounds with interesting activity (Fig. 3 & 4).
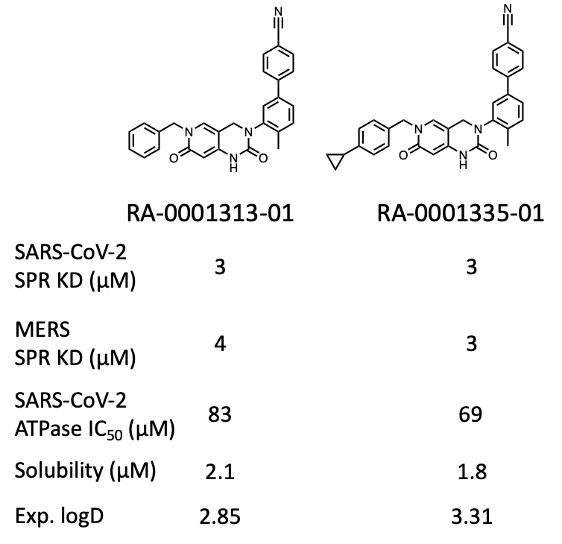
Figure 3: Activity profile of two representative analogs
Some weak activity showed up in the cellular antiviral assay (Fig. 5).
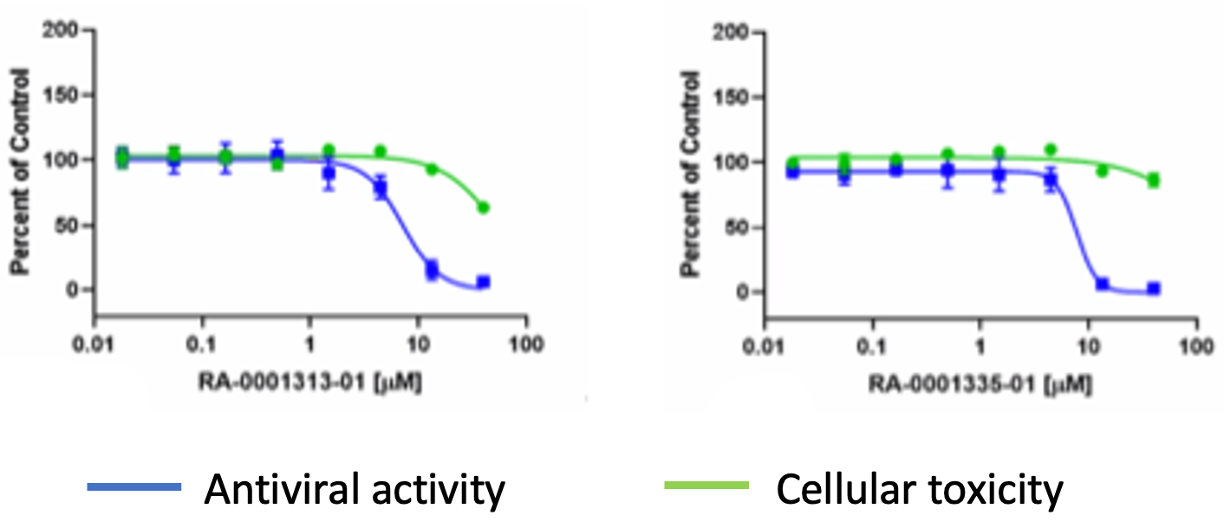
Figure 5: Antiviral activity of selected Brr2 analogs
We continue to develop the SAR of this series of compounds to find more potent inhibitors of SARS CoV-2 nsp13. The original paper describing the discovery of Brr2 inhibitors also reported a crystal structure of compound (1) bound to Brr2 (PDB: 5URM); however, this site is not present in the apo-structure of nsp13 (PDB: 7NIO).
Our other chemistry initiative: Thiazole series for helicase
To know more about READDI: https://www.readdi.org/
Interested in participating in AViDD Open Science? Check here
Acknowledgment:
- SPR and ATPase assay: Sumera Perveen, University of Toronto
- Antiviral Assay: Professor Sara Cherry, University of Pennsylvania
- Chemistry Synthesis: Piramal Pharma Solution
References:
- Iwatani-Yoshihara, M.; Ito, M.; Klein, M.G.; Yamamoto, T.; Yonemori, K.; Tanaka, T.; Miwa, M.; Morishita, D.; Endo, S.; Tjhen, R.; Qin, L.; Nakanishi, A.; Maezaki, H.; Kawamoto, T. Discovery of Allosteric Inhibitors Targeting the Spliceosomal RNA Helicase Brr2. Journal of medicinal chemistry 2017, 60, 5759-5771, doi:10.1021/acs.jmedchem.7b00461.
