To understand what proteins do, scientists often test the consequence their absence has on a cell. A common method uses a cells regulatory machinery to disrupt gene expression with complementary RNA molecules, known as RNA interference. Here I used small interfering RNAs (siRNAs) to deplete NSD3 and test the effect this has on epithelial cell identity in a lung cancer model. Importantly, siRNA molecules can be designed to target specific isoforms of NSD3, allowing us to test their individual functions (Figure 1 – Isoforms of NSD3).
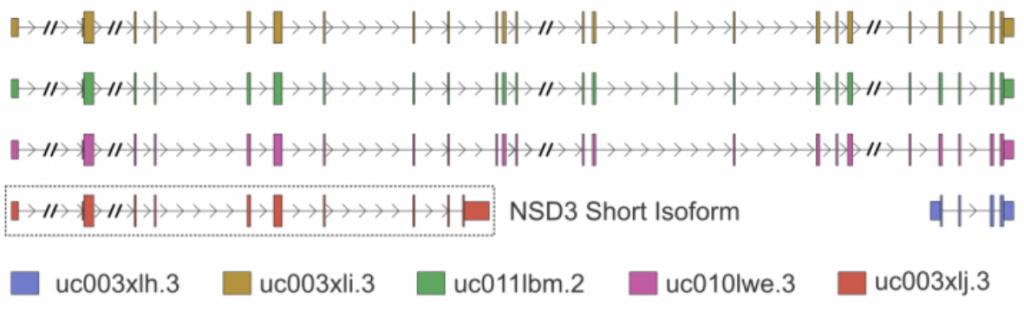
Figure 1. Gene-level depiction of known NSD3 isoforms.
I first tested several different concentrations of siRNA on H1299 cells and determined the degree of NSD3 loss by western blotting (Figure 2). Surprisingly, I observed depletion of NSD3 with only one of two antibodies tested (Figure 2, full experimental details). This suggests abcam’s NSD3 antibody (ab180500), which was advertised as knock-out validated, likely recognizes a completely different protein. Additionally, ab180500 did not recognize NSD3-Short, even though the epitope is present in the short isoform. This experiment shows how important it is to validate antibodies and that this reagent should not be used to study NSD3.
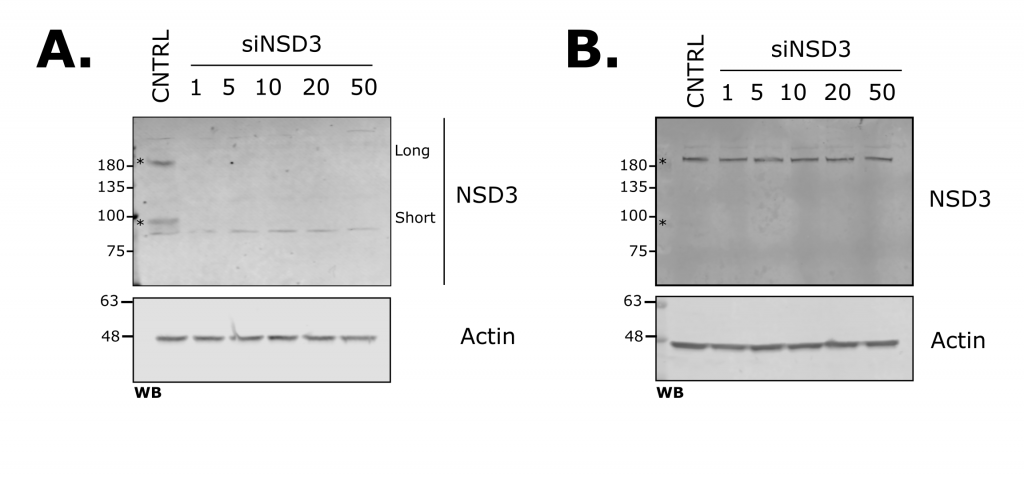
Figure 2. siRNA-mediated Knockdown of NSD3 in H1299 Lung Cancer Cells. Western blots show transfection with different nanomolar amounts of an NSD3-targeting siRNA probing with either A. anti-NSD3 (proteintech: 11345-1-AP) or B. anti-NSD3 (abcam: ab180500), with actin as a loading control.
Next, I looked at E-cadherin expression as a marker of an epithelial cell state (Figure 3, full experimental details). With siRNA that target both long and short isoforms, I observed an increase in E-cadherin when NSD3 was depleted. Previously I showed overexpression of NSD3 decreased E-cadherin, together these results suggest the amount of NSD3 present in a cell influences the transcriptional programs that control cell identity. To test if this function is isoform specific, I used siRNA molecules that target either the long or short isoforms of NSD3 (Figure 4, full experimental details – A, B). I found that knockdown of the short isoform and not the long increased E-cadherin expression levels and reduced cell migration. As the catalytic SET domain is absent in NSD3-short, NSD3’s H3K36me methyltransferase activity does not appear to be required for this function. Importantly, this supports the idea that NSD3-Short is an important mediator of cell identity and, in the context of cancer promotes, metastatic properties.
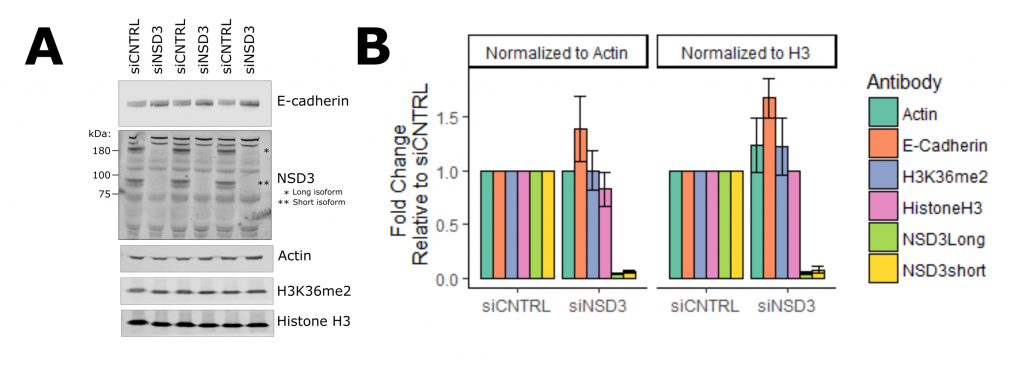
Figure 3. NSD3 Represses E-cadherin Expression in H1299 Lung Cancer Cells. A. Western blot of H1299 cells treated with NSD3-targeting siRNA in triplicate. B. Quantification of western blot signal relative to either actin or histone h3 loading controls.
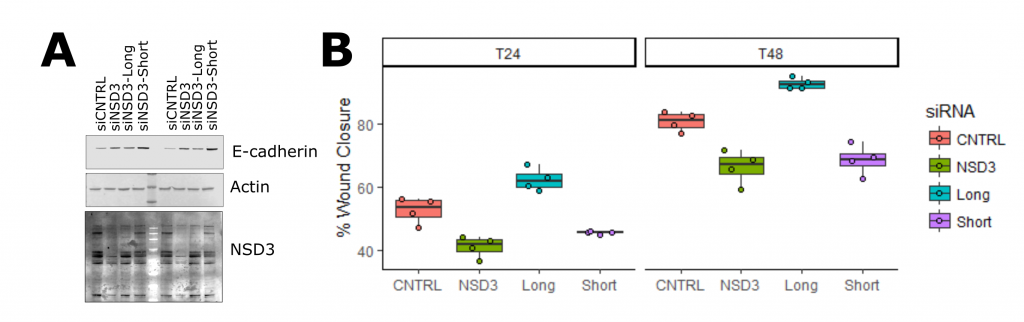
Figure 4. The short Isoform of NSD3 mediates its function in EMT. A. Western blot of E-cadherin protein levels in response to isoform-specific siRNAs in A549 lung cancer cells. B. Wound healing assay in A549 cells at 24 and 48 hours, indicates the short isoform of NSD3 is responsible for promoting migration in lung epithelial cells.
