Background
The Structural Genomics Consortium (SGC) has been funded by the Bill & Melinda Gates Foundation to research Women’s and Children’s Health, focusing on the advancement of drug discovery in reproductive biology and disease, child development, and childhood diseases. The SGC plans to generate protein reagents and chemical probes for several potential drug targets for new, safe and effective non-hormonal contraceptives.
Contraceptives allow women to make informed choices about family planning. Research into non-hormonal contraceptives has historically been neglected but is important due to concerns over the side effects of current hormonal contraceptives.
PLCζ1
One promising protein target is human phospholipase C zeta 1 (PLCζ1). PLCζ1 is found within the sperm and is known to be a sperm oocyte activation factor (SOAF). PLCζ1 catalyses the hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) into inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG).1,2
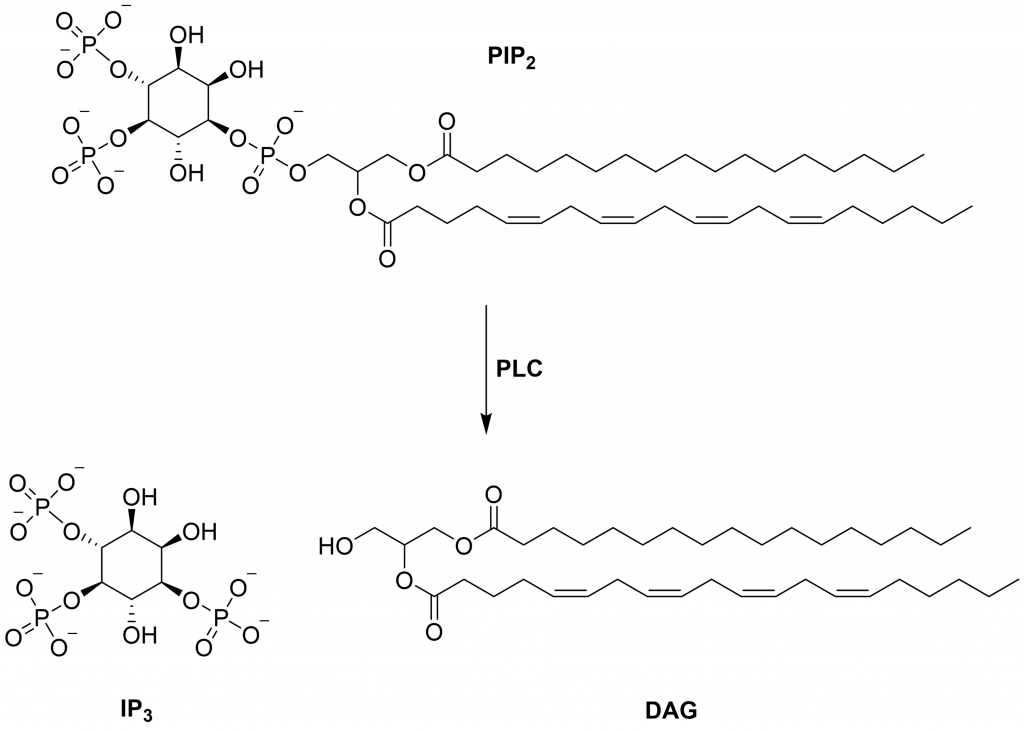
Figure 1: Hydrolysis of PIP2 into IP3 and DAG.
Once released, IP3 is then able to bind to its receptors (IP3R) located in specialised compartments of the endoplasmic reticulum (ER) membrane and release intracellular calcium (Ca2+) from the ER. These Ca2+ oscillations are vital for successful fertilisation.1,3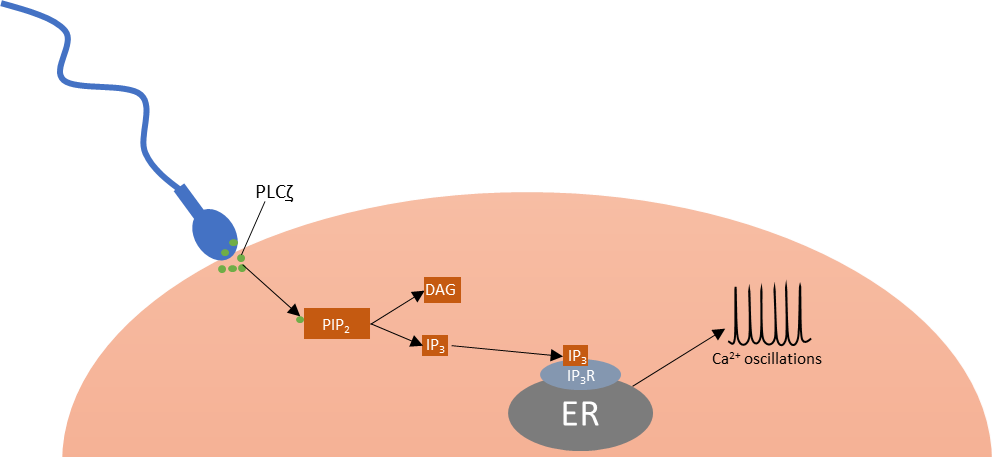
Figure 2: Action of PLCζ, releasing Ca2+ oscillations.
Absence or mutations of PLCζ1 in the sperm head has been associated with failure to signal Ca2+ oscillations and therefore fertilisation failure.4,5 PLCζ1 RNA and recombinant PLCζ1 directly injected into the oocyte cytoplasm were seen to induce Ca2+ oscillations similar to those observed during fertilization.4,6,7
The structure of PLCζ1 consists of four EF-hand domains, X and Y catalytic domains separated by a linker, and a C2 domain. The EF-hands are involved in Ca2+ binding, the X and Y catalytic domains are responsible for the hydrolysis of PIP2, and the C2 domain is thought to anchor the protein to intracellular vesicles.1
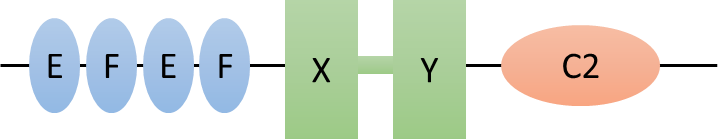
Figure 3: Structure of PLCζ1.
References
(1) Thanassoulas, A.; Swann, K.; Lai, F. A.; Nomikos, M. The Structure and Function Relationship of Sperm PLCZ1. Reproduction 2022, 164 (1), F1–F8. https://doi.org/10.1530/REP-21-0477.
(2) Nomikos, M.; Kashir, J.; Lai, F. A. The Role and Mechanism of Action of Sperm PLC-Zeta in Mammalian Fertilisation. Biochem. J. 2017, 474, 3659–3673. https://doi.org/10.1042/BCJ20160521.
(3) Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F. A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8 (28), 1–9. https://doi.org/10.3389/fcell.2020.00028.
(4) Zafar, M. I.; Lu, S.; Li, H. Sperm-Oocyte Interplay: An Overview of Spermatozoon’s Role in Oocyte Activation and Current Perspectives in Diagnosis and Fertility Treatment. Cell Biosci. 2021, 11 (4). https://doi.org/10.1186/s13578-020-00520-1.
(5) Yuan, P.; Zheng, L.; Liang, H.; Lin, Q.; Ou, S.; Zhu, Y.; Lai, L.; Zhang, Q.; He, Z.; Wang, W. Novel Mutations in the PLCZ1 Gene Associated with Human Low or Failed Fertilization. Mol. Genet. Genomic Med. 2020, 8 (10), e1470. https://doi.org/10.1002/mgg3.1470.
(6) Nomikos, M.; Yu, Y.; Elgmati, K.; Theodoridou, M.; Campbell, K.; Vassilakopoulou, V.; Zikos, C.; Livaniou, E.; Amso, N.; Nounesis, G.; Swann, K.; Lai, F. A. Phospholipase Cz Rescues Failed Oocyte Activation in a Prototype of Male Factor Infertility. Fertil. Steril. 2013, 99 (1), 76–85. https://doi.org/10.1016/j.fertnstert.2012.08.035.
(7) Barberán, A. C.; Boel, A.; Meerschaut, F. Vanden; Stoop, D.; Heindryckx, B. Fertilization Failure after Human ICSI and the Clinical Potential of PLCZ1. Reproduction 2022, 164 (1), F39–F51. https://doi.org/10.1530/REP -21-0387.
