Hello everybody! I am Sabrina, a third year DPhil student in Wyatt Yue’s lab at the SGC University of Oxford, co-supervised by Paul Brennan. I am fascinated by how a single base change in an enzyme-coding gene can lead to a diverse group of symptoms and how the severity of such diseases can be reduced by the provision of missing, or reduction of accumulating, components. My project aims to look at how these approaches can apply to developing therapy for metabolic disorders.
In this first post, I would like to share the background of my research project:
Primary hyperoxalurias are liver defects with kidney manifestations
Primary hyperoxalurias are rare genetic disorders caused by inherited mutations in one of three liver enzymes responsible for detoxifying glyoxylate, a normal metabolite produced during amino acid breakdown and production (figure 1). When glyoxylate is not metabolised, it accumulates and is converted to insoluble oxalate which is transported to the kidneys for excretion where it is deposited as calcium oxalate crystals (kidney stones), causing progressive kidney damage.
The most common and severe primary hyperoxaluria is type 1 (PH1), caused by mutations in alanine: glycine aminotransferase (AGXT), the liver-specific peroxisomal enzyme that converts glyoxylate and alanine to glycine and pyruvate. In patients with PH1, complete kidney failure generally occurs by 30 years of age, with a subset of patients (around 10%) reaching end stage renal failure in infancy.
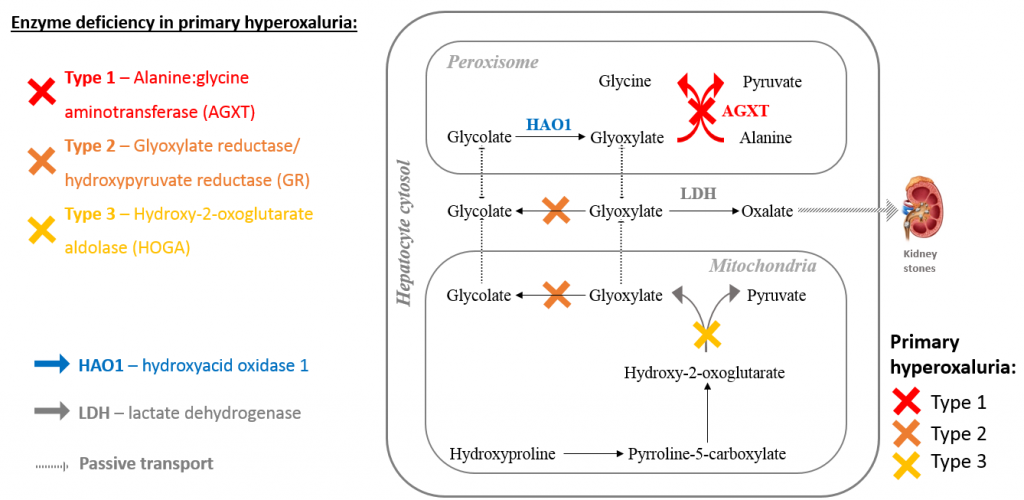
Figure 1: Types of primary hyperoxaluria and glyoxylate metabolism.
Substrate reduction therapy could address an unmet need for PH1
To date the only treatment able to permanently arrest the decline of kidney function in PH1 patients is a combined liver and kidney transplant. Given the limited availability and high associated morbidity of transplantation, alternative therapeutic approaches are desperately needed. We propose that a substrate reduction approach could meet this need.
The aim of substrate reduction therapy, as the name suggests, is to slow the formation of the substrate that accumulates in the disease state, by inhibiting an upstream enzyme. This concept has been neatly illustrated using the analogy of turning down a tap to avoid overflowing a slow draining sink (check it out here). In the 20 years since substrate reduction was first proposed for the treatment of Gaucher, a lysosomal storage disorder (Dr Norman Radin, 1996), four substrate reduction drugs have been approved for clinical use in rare metabolic diseases.
I believe that substrate reduction is an attractive approach for PH1, because the characteristic kidney damage is caused by accumulated glyoxylate, the substrate of the defective enzymes. In the peroxisome, glyoxylate is produced by the oxidation of glycolate by hydroxyacid oxidase 1 (HAO1), using FMN as a co-factor, and so inhibition of HAO1 should decrease glyoxylate, and subsequently oxalate, production (figure 2).
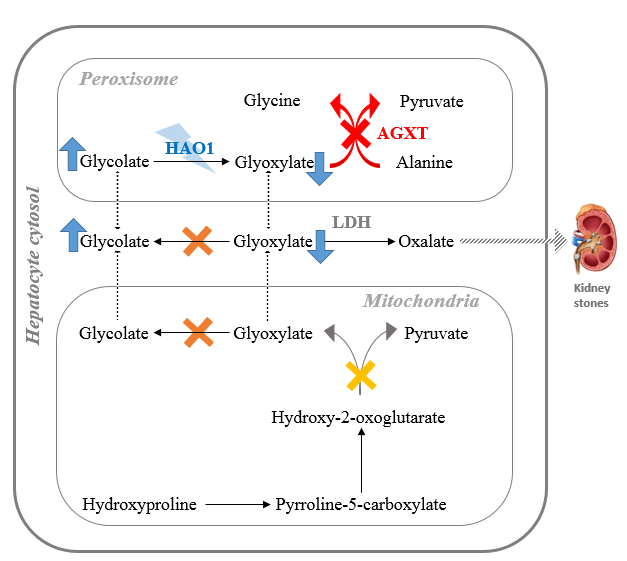
Figure 2: Potential for substrate reduction therapy to treat primary hyperoxaluria: Targeting HAO1 to reduce glyoxylate production in the peroxisome.
HAO1 is an excellent target for substrate reduction therapy because:
- Patients with inherited mutations reducing HAO1 function are asymptomatic i.e. HAO1 deficiency is a ‘non-disease’
- RNAi knockdown of HAO1 has been shown to decrease oxalate levels in PH1 animal models, and patients (clinical trial of ALN-GO is ongoing)
- The HAO1 protein has been well characterised biochemically and structurally over the past two decades
In fact, the first crystal structure of HAO1 was solved here at the SGC back in 2006, providing insight into the enzyme’s oligomeric state (tetrameric; figure 3A), co-factor binding (canonical FMN binding protein fold; figure 3B) and active site architecture (figure 3C).
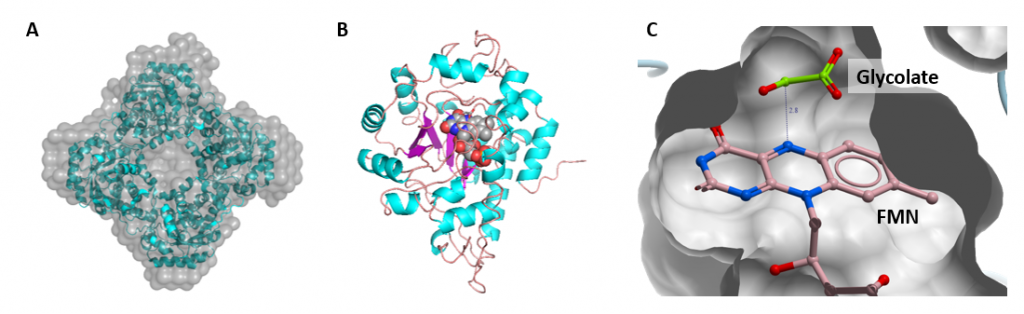
Figure 3: Structural features of HAO1. A. HAO1 forms a tetramer both in solution and in crystal form. B. Crystal structure of HAO1 monomer coloured by secondary structure – helices in blue, sheets in purple and loops in pink; FMN is shown as grey spheres. C. Close up from crystal structure showing active site pocket bound with FMN and glycolate bound.
In my subsequent posts, I will tell you about how I am combining cutting-edge methods of fragment screening, x-ray crystallography, and chemical biology to identify, characterise and validate HAO1 inhibitors.
