Acute myeloid leukemia or AML is a type of cancer that is the result of abnormal division and development of blood cells. AML has very poor outcomes, with 5-year survival of less than 50 percent and even less in patients over the age of 60 [1]. Within AML, there are many subgroups that can dictate prognosis and potential treatment options. A group of particularly poor prognosis patients are those with an inversion in chromosome 3 (denoted as inv(3)(q21q26.2) or t(3;3)(q21;q26.2)) [2]. The inversion causes activation of a cancer promoting gene, EVI1 (Figure 1) [3]. We have focused our study on understanding how EVI1 causes AML and identifying potential novel therapies for the inv(3) group because these poor prognosis patients do not respond to the current treatment options.
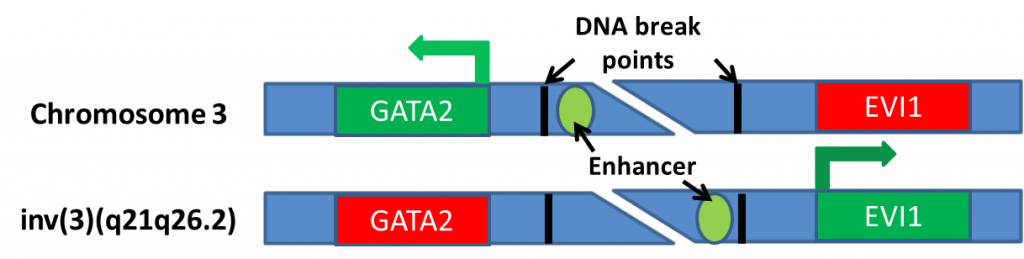
Figure 1: Inversion 3 causes an enhancer that normally allows the expression of GATA2 to rearrange, leading to inappropriate expression of EVI1.
Inv(3) can co-occur with a number of other mutations that contribute to the poor prognosis such as loss of chromosome 7. Chromosome 7 carries a number of genes and one of those genes is enhancer of zeste 2 (EZH2) [4] that functions as a methyltransferase in the polycomb repressive complex 2 (PRC2) (Figure 2). The PRC2 complex comprises of a number proteins that coordinate the regulation of gene expression by adding methyl groups at lysine 27 of histone 3 (H3K27) (Figure 2) [5]. It has been reported that EVI1 recruits PRC2 to repress an important tumor suppressor gene, PTEN [6].
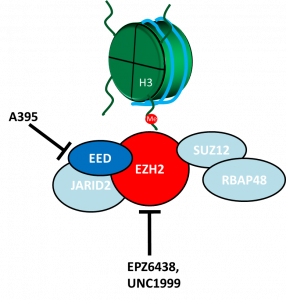
Figure 2: PRC2 complex is responsible for the methylation of lysine 27 on histone 3. Chemical probes/inhibitors to PRC2 (A395, UNC1999 and EPZ6438) will be used in this study.
We are interested in how the oncogenic protein EVI1 interacts with the PRC2 complex components and how modulating the PRC2 by using chemical probes affects leukemia. This blog will follow our experiments to further examine the PRC2 and EVI1 interactions in inv(3) AML with or without deletion of chromosome 7, elucidate the importance of these molecules in leukemia cell survival and finally investigate the therapeutic potential of targeting these complexes to cure inv(3) AML.
- Gregory, T.K., et al., Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J Hematol Oncol, 2009. 2: p. 23.
- Lugthart, S., et al., Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol, 2010. 28(24): p. 3890-8.
- Glass, C., et al., The role of EVI1 in myeloid malignancies. Blood Cells Mol Dis, 2014. 53(1-2): p. 67-76.
- Lavallee, V.P., et al., EVI1-rearranged acute myeloid leukemias are characterized by distinct molecular alterations. Blood, 2015. 125(1): p. 140-3.
- Greenblatt, S.M. and S.D. Nimer, Chromatin modifiers and the promise of epigenetic therapy in acute leukemia. Leukemia, 2014. 28(7): p. 1396-406.
- Yoshimi, A., et al., Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood, 2011. 117(13): p. 3617-28.

One Reply to “Project Overview: Testing polycomb repressive complex 2 (PRC2) inhibitors in acute myeloid leukemia”