I am very excited to write my first post in open notebook. My post on Zenodo summarizes the results of my first ever purification of a huntingtin (HTT) domain. I was able to purify successfully the HTT C-HEAT domain encompassing residues 2088-3144.
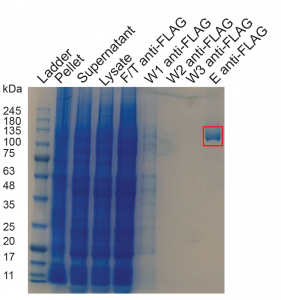
Figure 1. Anti-FLAG purification of the HTT C-HEAT domain. Eluted protein is seen in the red box!
This purification was first done by Dr. Rachel Harding (Zenodo). Following her purification protocol, I obtained good yields of protein (0.45 mg of protein per L of Sp9 cells) which I immediately used to set crystal trays and to initiate biophysical characterization.
In future purifications, my aim is to improve purity as I noticed the sample was not very clean (low molecular weight proteins and nucleic acid). Further, I want to do a more stringent analysis to determine the most ideal buffer conditions for the sample using DSLS and DSF. I also would like to use SDS-PAGE and native PAGE to determine the progression of the limited proteolysis over time.
