His6-FLAG-ABHD233-425 (PBC042 A11) Purification: Nickel & Size Exclusion Chromatography
This post is also available at: Recombinant Expression and Purification of ABHD2 from E. coli | Zenodo
DOI: 10.5281/zenodo.7696914
For relevant background please see relevant page: A promising target for non-hormonal contraception – The a/b Hydrolase Domain 2 | Zenodo
Expression of ABHD2
- For each 1.8 L of culture, you will need 25 mL of overnight culture. So, for 10 L prepare 200 mL of LB, add 200 μL of 1000X Chloramphenicol (CAM) and of 1000X Kanamycin (KAN). Inoculate from glycerol stock.
- The next day. To each 1.8 L bottle of TB with glycerol add:
- 1 mL of anti-foam
- 8 mL of 1000X Chloramphenicol
- 8 mL of 1000X Kanamycin
- 25 mL of overnight culture
- Add glycerol if it was not previously added to the Terrific Broth
- Grow cells until the OD600 reaches 0.9-1.4. Save an uninduced sample if needed (spin down cells from 500 μL).
- Cool the cells to 15°C by adjusting the temperature on the water bath. After 20 minutes of letting the cells cool at 15°C, induce protein expression by adding 540 μL of 1 M IPTG to each 1.8 L of culture for a final concentration of 0.3 mM. Let cells induce for 18 hours at 15°C.
ABHD2 Purification
- The next day, harvest cells by centrifuging at 6,000 x g for 15 minutes.
- Resuspend cells in Sonication Buffer. You will need ~300 mL for pellets from 10 L of growth.
- If 8 L or more of cells, sonicate the cells for 7 minutes (total sonication time) with 8 seconds on, 30 seconds off. Power level 8, which should reach 90 W.
- Add 10 μL of Benzonase (0.3 mg/mL stock from fridge) and 1 M MgCl2 (2 mM final conc) was added for every ~100 ml of cell lysate. Let incubate with stirring for 10 minutes.
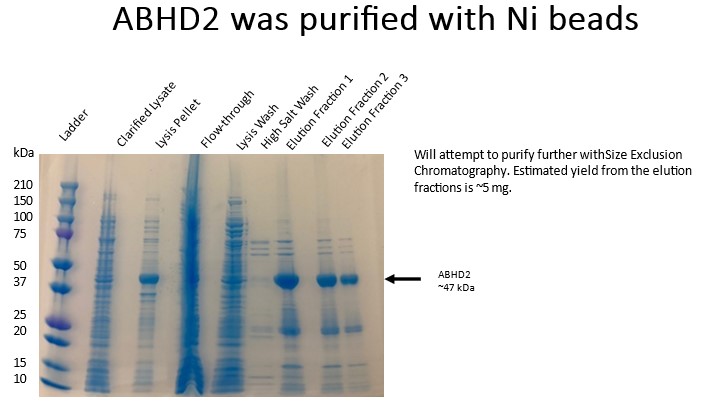
- Cell debris was removed by centrifugation at 29,000 x g for 50 min. Save a sample for gel from supernatant and pellet.
- Meanwhile, equilibrate Nickel column with Lysis buffer supplemented to be 10 mM Imidazole. Use at least 5 mL of beads for 10 L of growth.
- Incubate the clarified lysate (supplemented with imidazole to 10 mM) for 1 hour with Nickel resin for batch binding. Make sure to add imidazole to the clarified lysate!
- Centrifuge the batch bind at 700 × g at 4 °C for 5 min then discard the supernatant (flow-through). Save a sample for gel.
- Beads were loaded on a gravity flow column and washed with 20 CV Lysis buffer, 40 CV Wash Buffer, then 4 CV Lysis buffer to lower salt concentration. Save a sample for gel.
- Elute with addition of Elution buffer (1 CV). Let buffer sit for 10 min with beads before collecting. Rinse beads with additional 2 CVs and save these elutions as well. Repeat if necessary. Check concentrations of elution fractions to decide which to save. Save samples for gel.
- Run samples on a 4 – 20% gel to check samples: 230 V, 42 min.
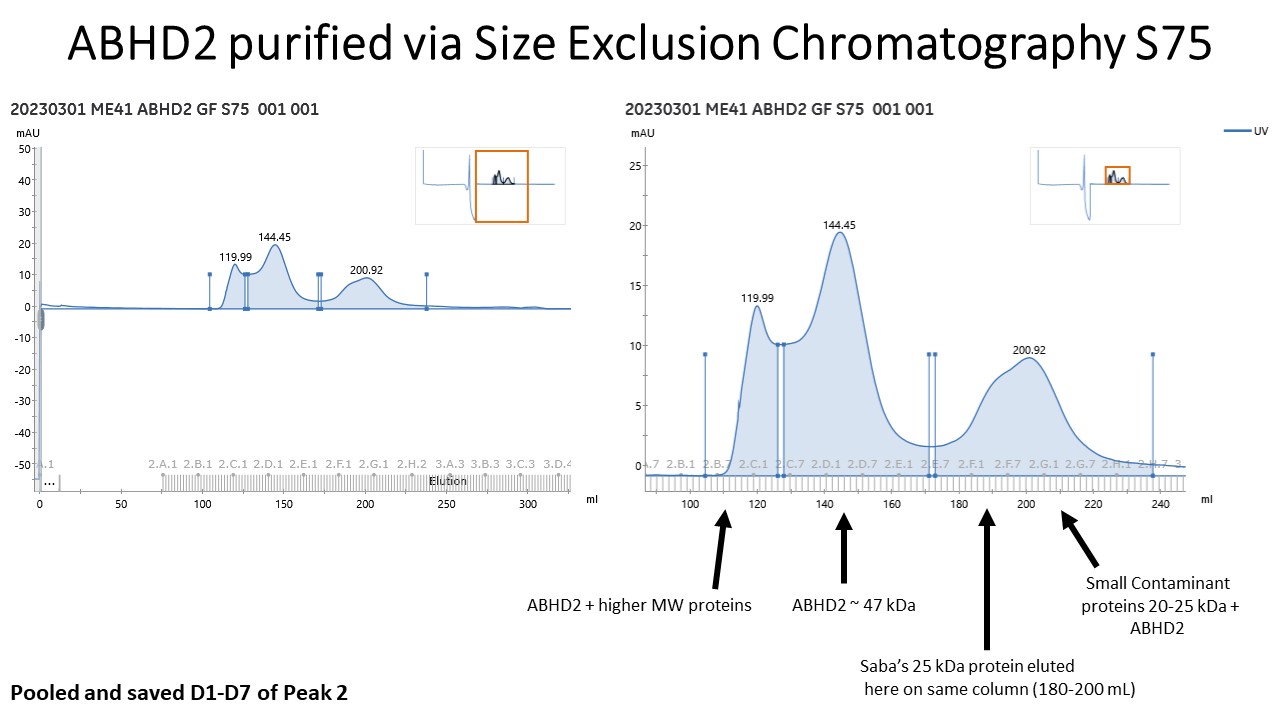
- Meanwhile, prepare the Size Exclusion Column (Hiload 26/600 Superdex 75 pg) to equilibrate with Size Exclusion Buffer.
- Decide which samples to pool and concentrate if necessary. For a 300 mL column, volume of sample loaded should be 10 mL or less. If concentrating, use an equilibrated 15 mL, 10 kDa centrifugal filter. Perform 8 min spins in a swinging bucket centrifuge at 2,400 x g. Pipet in between spins.
- After concentrating, filter protein through a 0.2 μm filter before adding into a 50 mL falcon tube for loading onto the column. Save samples for gel.
- After SEC, look at elution chromatogram and run another protein gel to determine which samples to concentrate. In the meantime, equilibrate more 15 mL, 10 kDa centrifugal filters if need to concentrate.
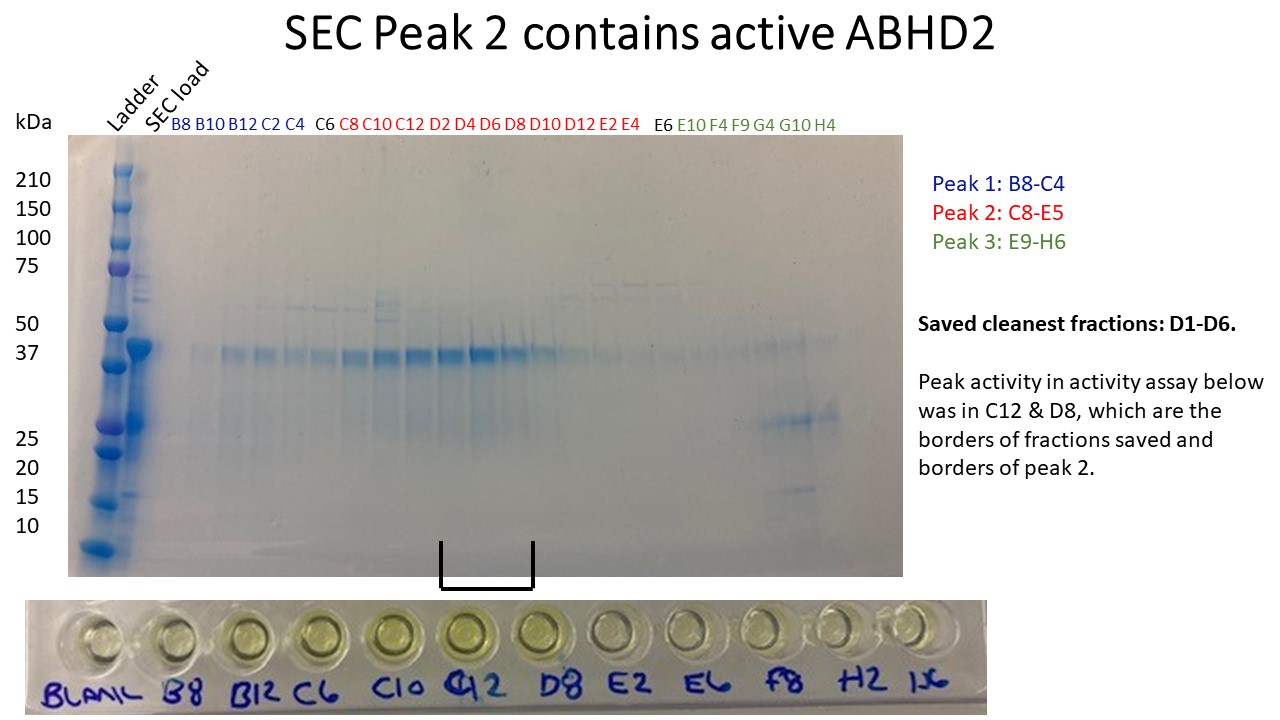
- At this stage it would also be good to perform an activity assay for the SEC fractions to determine which fractions have peak activity. This should correspond with where ABHD2 eluted from the column.
- Pool fractions and concentrate with an equilibrated 15 mL, 10 kDa centrifugal filter. Perform 8 min spins in a swinging bucket centrifuge at 2,400 x g. Pipet in between spins. Save a sample for gel.
- Prior to storing sample, centrifuge 10 min at 16,000 x g in a tabletop centrifuge to remove any aggregated protein. Measure final concentration of sample and determine volume to calculate the yield. A pure protein sample will have an A260/280 value under 0.6. Save a sample for gel.
- Freeze samples in liquid nitrogen and store at -80°C.
- Run a final gel to analyze the final stored samples.
Buffers *Add right before use.
Lysis Buffer (1L)
- 50 mM Tris pH 8 (50 mL of 1M)
- 500 mM NaCl (100 mL of 5M)
- 10% Glycerol (100 mL)
- 1 mM TCEP (2 mL of 0.5 M)*
Sonication Buffer (300 mL)
- Lysis Buffer
- 1% Triton X (3 mL of 100%)
Wash Buffer (500 mL)
- 50 mM Tris pH 8 (25 mL of 1M)
- 30 mM Imidazole (3 mL of 5 M)
- 1 M NaCl (100 mL of 5 M)
- 1 mM TCEP (1 mL of 0.5 M)*
Elution Buffer (50 mL)
- 50 mM Tris pH 8 (2.5 mL of 1M)
- 250 mM Imidazole (2.5 mL of 5 M)
- 500 mM NaCl (5 mL of 5 M)
- 10% glycerol (5 mL)
- 1 mM TCEP (100 μL of 0.5 M)*
Size Exclusion Buffer (2 L) (filter buffer before using with FPLC)
- 50 mM Tris pH 8 (100 mL of 1M)
- 500 mM NaCl (200 mL of 5 M)
- 10% glycerol (200 mL)
- 1 mM TCEP (4 mL of 0.5 M)*
Extinction Coefficients & Molecular Weight
His6-FLAG-ABHD233-425 (PBC042 A11)
- εRED: 58,790 M-1 cm-1 or 1.254 g/L
- MW= 47 kDa
- pI 6.25