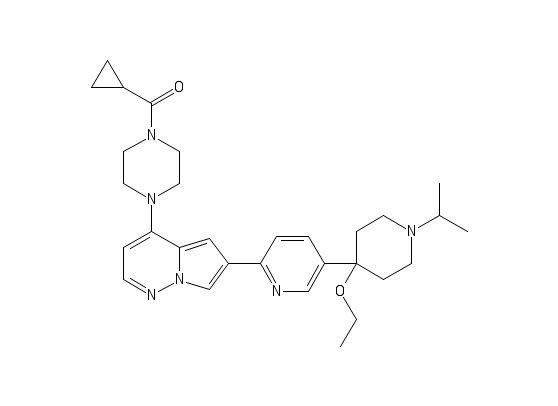
M4K3007
In the absence of our “workhorse” protein, ACVR1 residues 208-499 (constitutively active Q207D mutant) (as we are still trying to work out why it won’t express at the moment), and the complete lack of crystals/diffracting crystals for the TGFBR1/FKBP12 complex, I have shifted to some other constructs we have to keep things moving. I had some purified short form R206H ACVR1 mutant (without the FKBP12 binding site) in the freezer, so I ran it over the gel filtration column again and set up crystal plates with M4K2009 and M4K3007. A few small, ugly crystals grew, which I screened at the synchrotron, but they didn’t diffract. I have another 2L pellet of this construct in my freezer, so will purify more and try it with some of the other compounds.
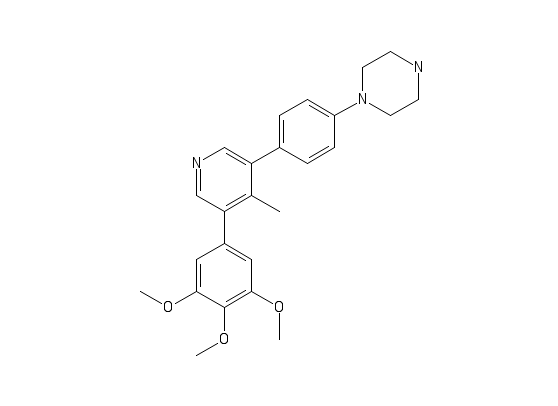
M4K2009
More recently (last week), I purified a shorter form of TGFBR1 (ALK5) (residues 198-503, instead of the longer form I’ve been using with the FKBP12 binding site, residues 162-503). Experimental details and compound structures are available here. This particular construct hasn’t been used in a while, but it expressed ok (baculo). The ultimate yield wasn’t fantastic (around 2.5 mg from 2 L cells), because it had to go through a nickel rebind step, increasing losses. I was able to set up 9 crystal plates with it, with 2 different compounds and 5 different coarse screens. Day 1 images show no crystals yet, but it’s still early days.
This week I intend to purify the long form Q207D ACVR1 mutant, and combine it with FKBP12 to screen for possible fragment screening conditions (since the synchrotron summer shutdown will be over next month), as well as co-crystallise more of the M4K compounds.
