Continuing with our goal to synthesize three compounds as ALK2 inhibitors, let’s refer back to Scheme 2 in the previous post on this topic, compounds 6, 8 and 9 were synthesized in good yields (65-90%), however compound 10 could not be obtained.
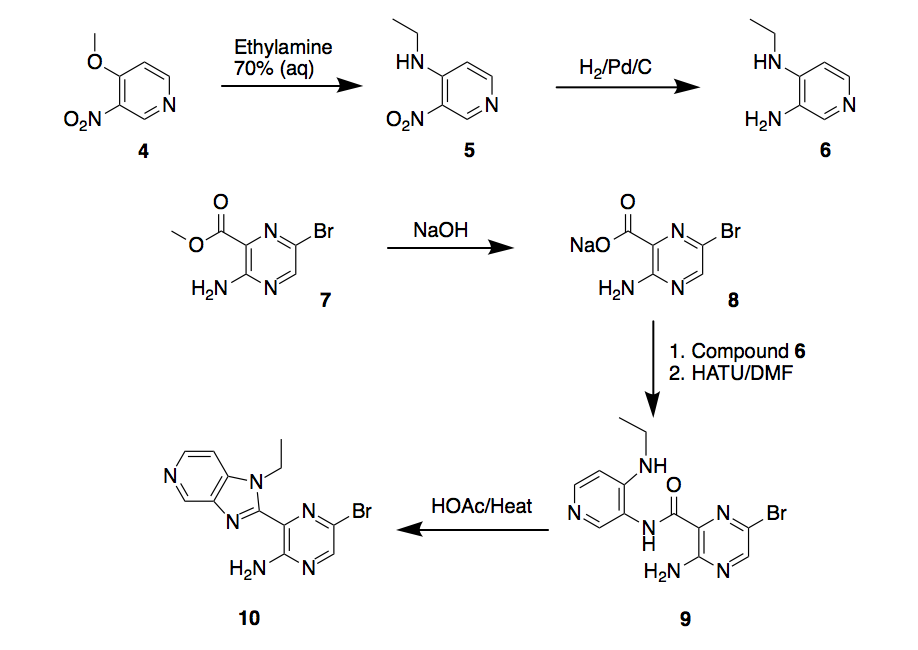
Scheme 2. Synthesis route 1
Different conditions were tested to induce the cyclization of the bromo-pyrazine-2-carboxamide 9 to the target bromo-pyrazin-2-amine 10. This reaction mostly yielded starting material 9 and by-product 11 in low yields (<15%). After several attempts, this approach (acid/heat) was abandoned.
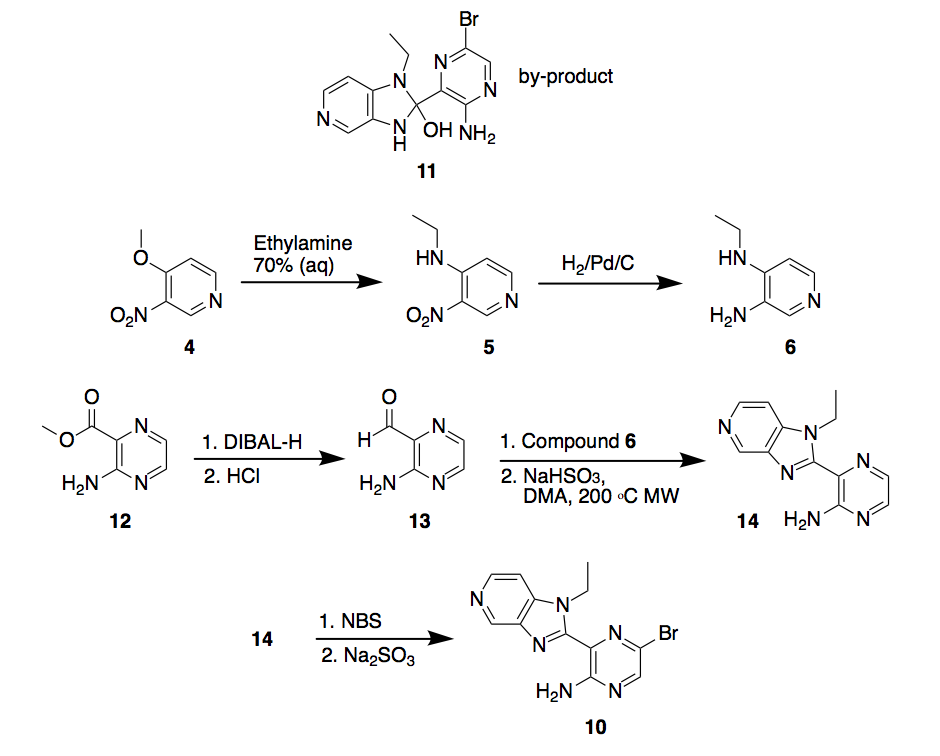
Scheme 3. Synthesis route 2
An alternative synthetic route is shown in Scheme 3. Here, aminopirazine 12 will be reduced with diisobutylaluminium hydride (DIBAL-H) to yield carbaldehyde 13, which then will be reacted with diamine 6 (previously synthesized) at 200 oC using dimethylacetamide (DMA) as a solvent. This last step can be carried out in a microwave reactor and should yield the cyclized compound pyridinpyrazin-2-amine 14, which can be brominated with N-Bromosuccinimide (NBS) to yield compound 10.
As it was mentioned before, 10 could not be synthesized by the previously proposed route. The new route is adapted from international patent WO 2008/071937 and we hope in this way we can access to this key intermediate in 150-200 mg scale to move forward with more reactions.
