Background:
Evaluation of the efficacy of M4K compounds in DIPG patient-derived cell lines is essential before any promising compounds can be further tested in mouse xenograft models. This approach can aid in narrowing down clinical compound candidates and reduce the time, resources and animal sacrifice needed downstream.
A robust and efficient readout for the changes in the viability of the DIPG cells needs to be established before it can be used to evaluate the M4K compounds. In addition, the amount of cells to be seeded at the beginning of the experiment has to be optimised to avoid overcrowding and starvation of the cells after extended culture times. Overcrowding and starvation will lead to increased cell death and prevent accurate estimation of the potency of M4K compounds (EC50).
Experimental design:
The wells of 96-well plate with transparent bottom will be coated with laminin to synchronise the mode of growth (adherent or non-adherent) among the different patient-derived lines. Additionally, this will promote even distribution of cells and allow most cells to be in focus when images are taken.
An increasing cell number (125 to 20000) will be seeded into each well. The amount of cells after 7 days of growth in 100µl of medium will be measured based on the area occupied and the total amount of ATP present in each well. In addition, propidium iodide which stains dead cells will be added at the beginning the experiment. At seeding number that are not saturating, the amount of cells after 7 days should remain proportional to the initial seeding number.
Results:
Timeline of experiment
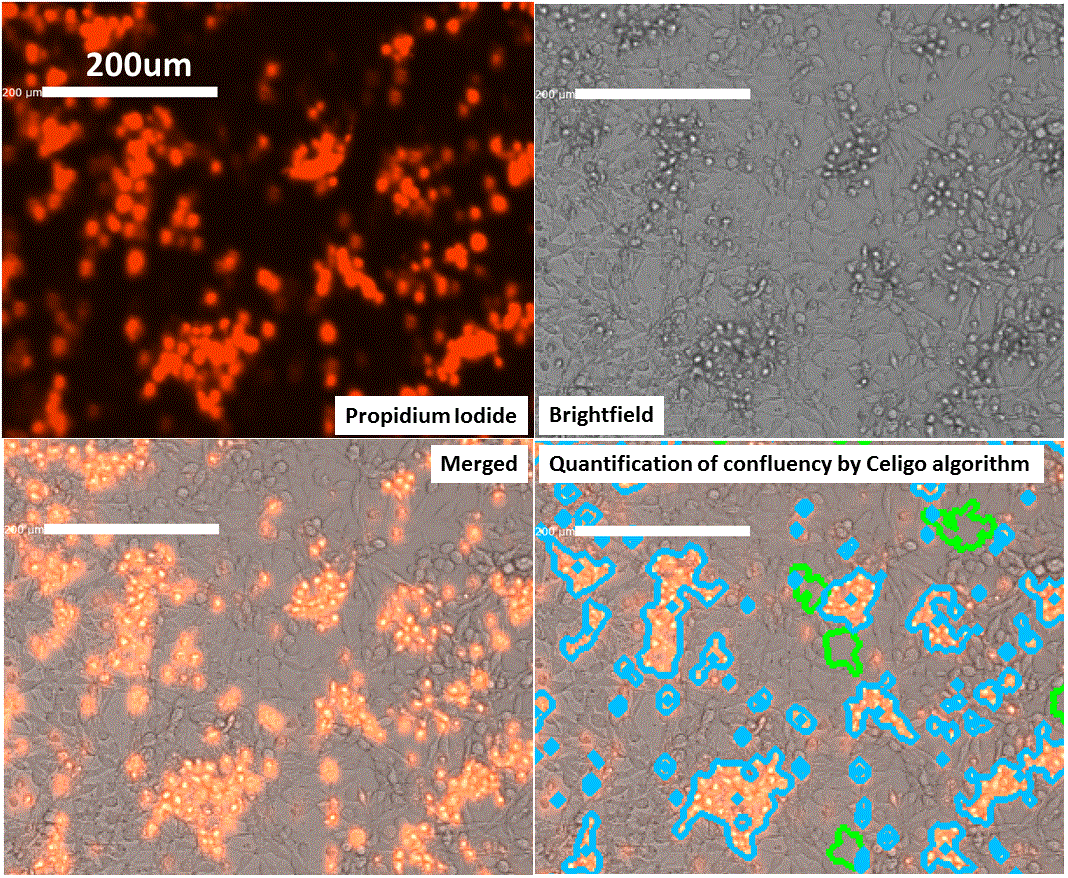
Demonstration of how Celigo algorithm quantifies the area occupied by total cells and area occupied by propidium iodide signal. Images of HSJD-DIPG-007 cells after 7 days in culture were used.
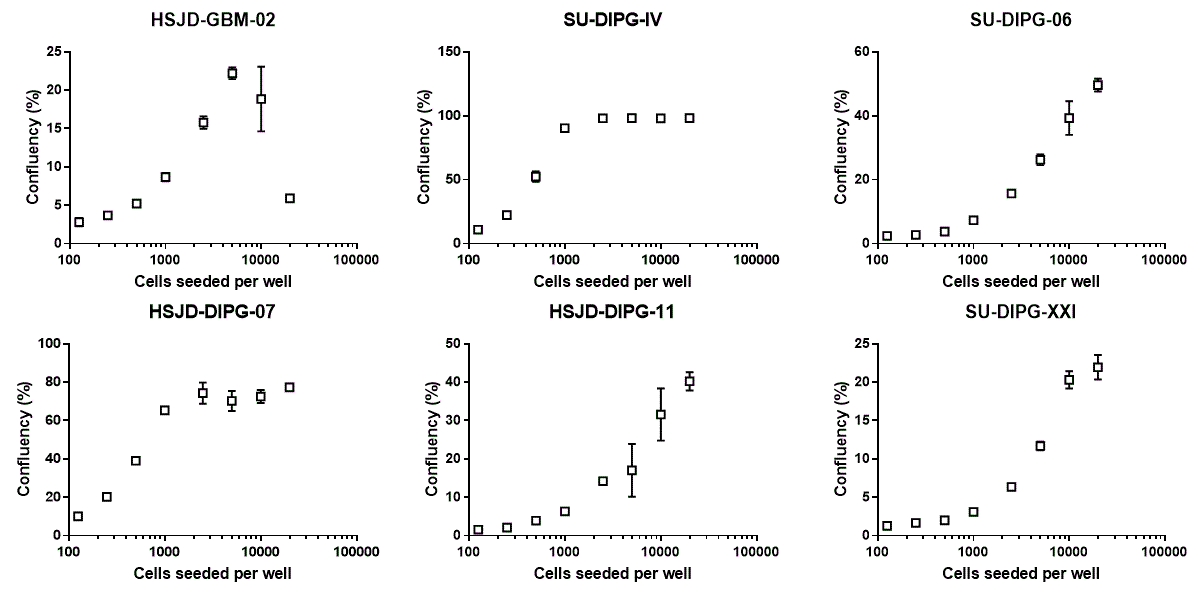
Percentage of area occupied by cells after 7 days in culture.
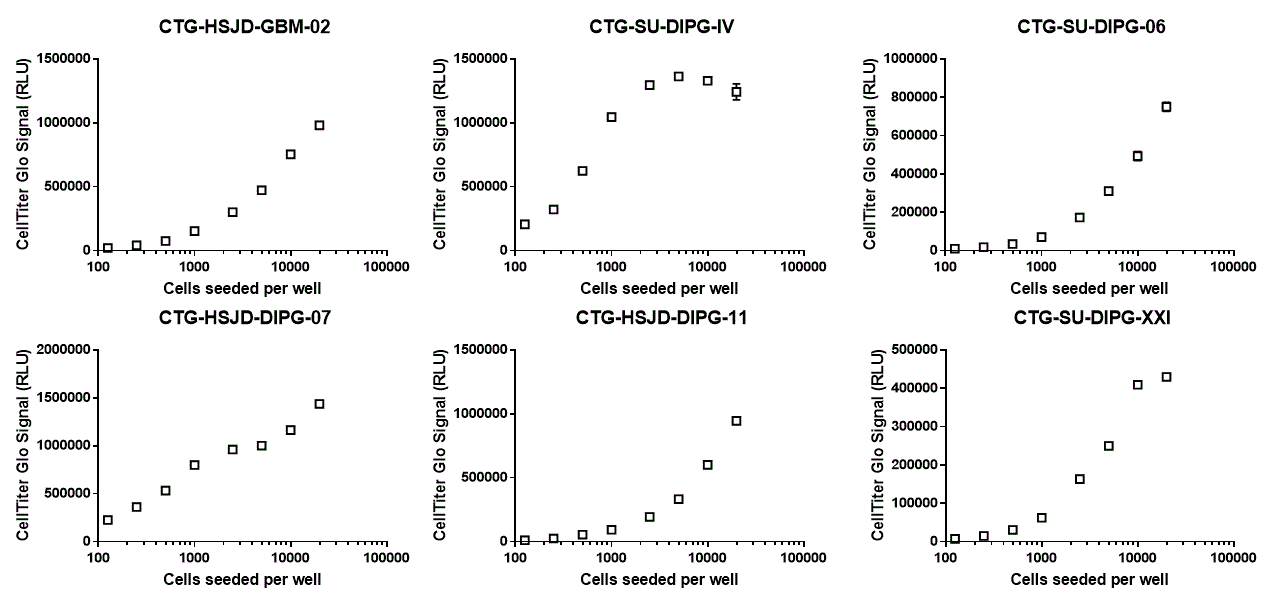
Luminescence signal from CellTiter-Glo at endpoint. ATP content is proportional to the amount of luminescence signal and is an indicator of cell viability.
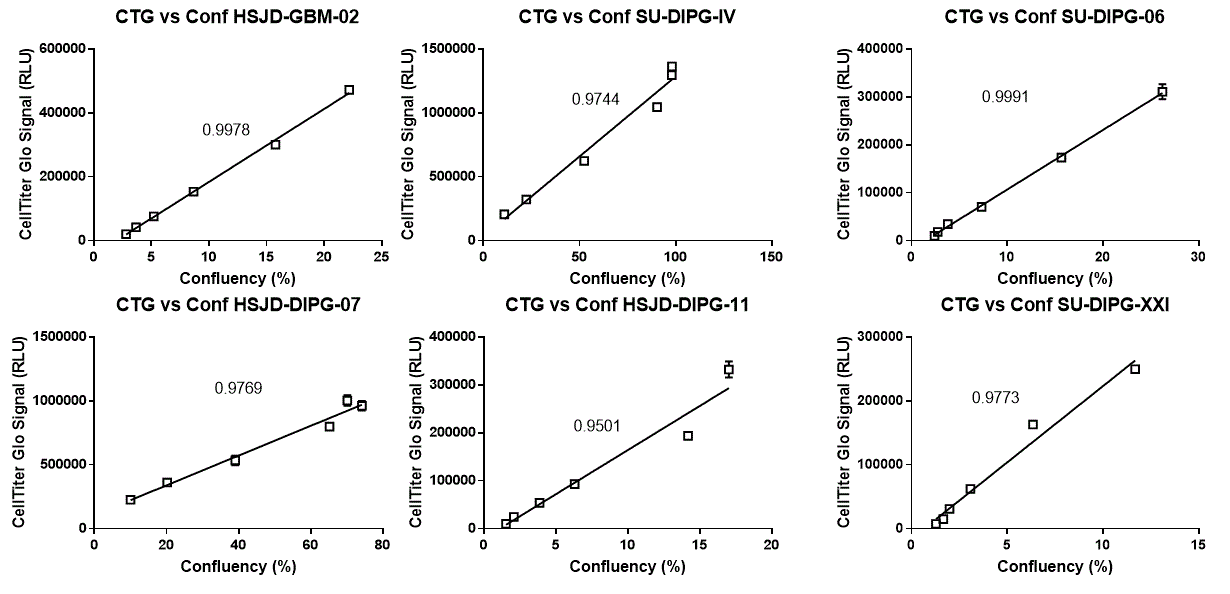
Near perfect linearity. The viability of the cells (CellTiter Glo signal) correlates almost perfectly with the area occupied by the cells (confluency) (below 5000 cells seeded per well). R squared values of the two measurements are indicated next to the fitted curves. A perfect correlation will have an R squared value of 1.
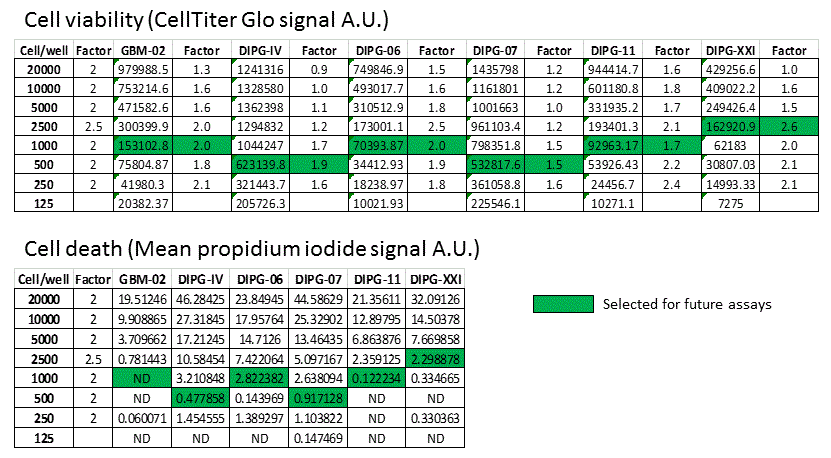
DIPG cell seeding numbers selected for future experiments
Findings:
1) Celigo and its algorithm is able to quantify the area occupied by the cells accurately.
2) SU-DIPG-IV and HSJD-DIPG-007 proliferate quickly. Seeding too many of these cells at the beginning of the experiment will saturate both confluency and CellTiter Glo readouts.
3) CellTiter Glo signal and confluency are good indicators of the actual quantity of cells in the well. Below 5000 cells seeded per well, both sets of measurements correlated almost perfectly with each other.
4) Cell death (as indicated by propidium iodide signal) in relation to total cell at endpoint increases when too many cells were seeded at the beginning of the experiment.
5) Initial cell seeding numbers for each patient-derived cell lines that do not lead to overcrowding at the end of the experiment can be chosen based on the linearity of CellTiter Glo signal and the intensity of propidium iodide.
In a nut shell:
We now have the parameters to go ahead and evaluate how well M4K compounds can stop the growth or kill DIPG cells.
For detailed experimental descriptions, please visit my Zenodo page.
