In a collaboration between SGC-University of North Carolina-Chapel Hill and M4K Pharma, we plan to synthesize 3 different imidazopyridin pyrazines as promiscuous kinase ALK2 inhibitors, compounds 1-3, Scheme 1.
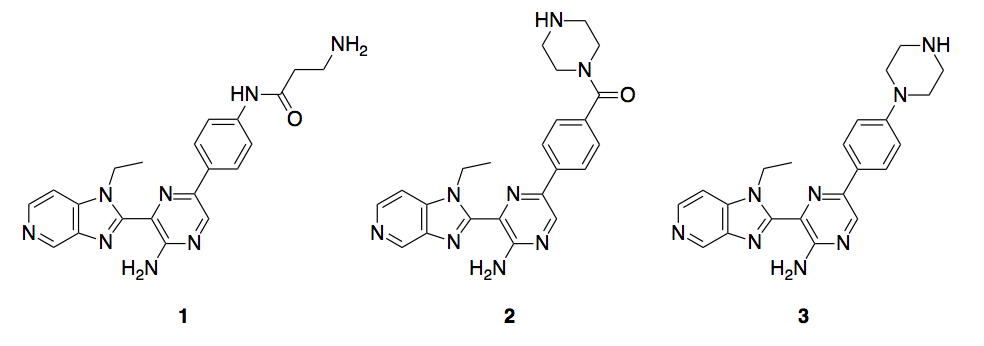
Scheme 1. ALK2 inhibitors
The first step will be the synthesis of key intermediate 10, Scheme 2. The proposed synthesis begins with a nucleophilic aromatic substitution on compound 4 to get nitropyridine 5, then reduction with hydrogen on palladium/carbon to obtain the pyridine-3,4-diamine 6.
On the other hand, aminopyrazine 7 will be saponified to the aminopyrazine sodium salt 8, which will be coupled with compound 6 using peptide coupling reagent HATU to form bromopyrazine carboxamide 9, followed by heating in presence of acetic acid to yield the cyclized intermediate 5-bromo-3-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)pyrazin-2-amine 10.
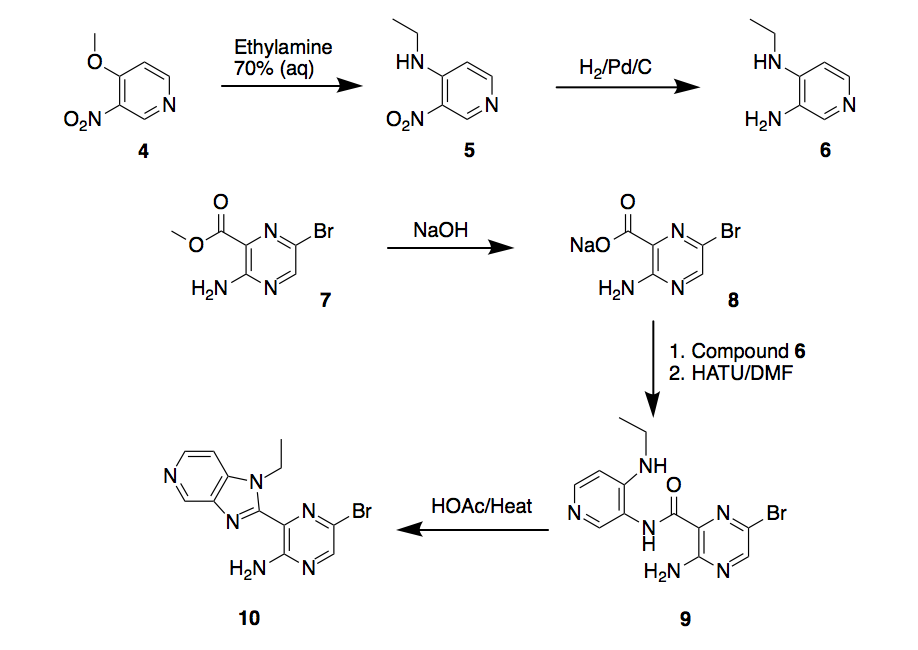
Scheme 2. Synthesis route 1
If we succeed with the proposed synthesis of imidazopyridine pyrazine 10, then soon we will present next steps to get to target compounds 1-3
This collaboration is the under supervision of Dr. David Drewry.
Synthesis routes were adapted from international patents WO 2006/063167 A1, WO 2008/071937 and ACS Med. Chem. Lett. 2016, 7, 5, 514-519.
