I used a medium-throughput 19F NMR assay to screen my second batch of compounds against USP5 zinc finger ubiquitin binding domain (Zf-UBD). To recap, the 19F NMR assay detects ligand binding by measuring perturbations in the resonance of a fluorinated tryptophan at the binding pocket. I selected the compounds for my second screen from SGC’s in house database, based on the structure of previous hit compounds; specifically, compounds that contained an aromatic ring and a propanoate moiety. You can find details of the NMR screening results on Zenodo.
As seen previously, control fluorinated USP5 Zf-UBD spectra has two peaks of equal intensity at approximately 118 and 125 ppm. Upon ligand binding, peak 1, corresponding to the tryptophan lining the ubiquitin binding pocket, undergoes a chemical shift to the right and a decrease in peak area (Figure 1). From the 28 compounds screened, 16 showed a significant decrease in peak area and chemical shift of peak 1. Once again, the high hit rate can be attributed to the high compound concentrated used (1 mM) in the 19F NMR assay. As this assay is more qualitative than quantitative, next I will be using a SPR assay to determine the binding affinity of the hits from the 19F NMR screen. I will also see if I can decrease compound concentration in future 19F NMR screens.
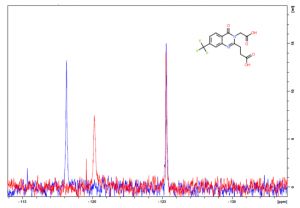
Figure 1. 19F NMR spectra of 40 µM USP5171-290 without (blue) and with (red) 1 mM UBXML78
