Background
PRDM9 (PR domain-containing protein 9) contains a subclass of the SET (PR/SET) domain that trimethylates histone 3 on lysine 4 and 36 (PIMID:24095733). It is expressed mainly in germ cells playing an important role in meiotic recombination (PIMID:27362481). Its aberrant expression has also been associated with genomic instability in cancer (PIMID:30341163).
Assay Validation
Consistent with previous findings, the overexpression of FLAG-tagged wild type PRDM9 but not its catalytic mutant (Y357A) led to an increase in histone 3 lysine 4 trimethylation levels (H3K4me3) of exogenous (GFP-tagged) as well as endogenous histone H3 in cells (Fig.1). Due to high basal H3K4me3 levels, only a small increase in methylation was observed upon PRDM9 overexpression in endogenous H3, making the system with exogenous histone H3 preferential assay for compounds screening. The assay was additionally validated with PRDM9 selective inhibitor MRK-740 (PIMID:31848333). MRK-740 decreased PRDM9 dependent H3K4 trimethylation of exogenous histone H3 in a dose-dependent manner (PIMID:31848333, Fig.2). The assay z factor equals 0.83.
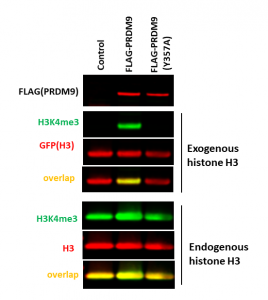
Fig.1. The wild type but not catalytic mutant of PRDM9 methylated exogenous and endogenous histone H3K4. HEK293T cells were co-transfected with GFP-tagged histone H3 and empty vector(control), FLAG-tagged wild type, or Y357A catalytic mutant PRDM9 24 h. The methylation levels were analyzed in Western Blot. (Shawna Organ)

Fig.2. MRK-740 decreases PRDM9 dependent H3K4 trimethylation in cells. HEK293T cells were co-transfected with FLAG-tagged PRMT9 (wild type or Y357A mutant) and GFP-tagged histone H3 and treated with inhibitor for 20 h. H3K4me3 levels were determined by Western blot. The graph represents the nonlinear fit of H3K4me3 signal intensities normalized to GFP. The results are mean +/- SEM of 3 replicates. (Magdalena Szewczyk)
Experimental details are posted on Zenodo.
