Background
SMYD2 is protein-lysine N-methyltransferase that methylates both histones and non-histone proteins, including p53. It monomethylates K370 of p53 leading to decreased DNA-binding and consequently the lower transcriptional regulation activity of p53 (PIMID:17108971).
Assay validation
Based on the findings (PIMID:17108971), we developed SMYD2 cellular assay in which exogenous FLAG-tagged p53 is methylated by exogenous V5-tagged SMYD2. The assay was validated with two SMYD2 selective chemical probes: BAY-598 and PFI-5 (PIMID: 27075367, 31415173). Both compounds decreased monomethylation of exogenous p53-K370 in cells overexpressed with V5-SMYD2 (Fig.1,2). The z factor for the assay equals 0.87 (n=3).
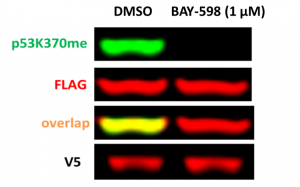
Fig.1. BAY-598 decreases SMYD2 dependent p53K370 monomethylation in cells. HEK293T cells were co-transfected with V5-tagged SMYD2 and FLAG-tagged p53 and treated with 1 µM BAY-598 for 20 h. The monomethylation levels of p53K370 were analyzed in Western blot.

Fig.2. PFI-5 decreases SMYD2 dependent p53K370 monomethylation in cells in a dose-dependent manner. MCF7 cells were co-transfected with V5-tagged SMYD2 and FLAG-tagged p53 and treated with PFI-5 for 20 h. The monomethylation levels of p53K370 were analyzed in Western Blot. The graph represents the nonlinear fit of p53K370me signal intensities normalized to p53(FLAG). The results are mean +/- SEM of 3 replicates.
Go to Zenodo for experimental details.
