My main project revolves around the E3 ligase UBE3A (I will be writing a project overview for this later), but I am also continuing some work in the same vein as Jolene Ho’s work looking at role of HTT in neurological disorders.
HTT encodes a protein called huntingtin whose function is not known but appears to be crucial to neuron function. Mutant HTT is frequently associated with Huntington’s Disease (HD), where the 5′ end of the HTT gene is observed to have an expanded CAG repeat region – the trinucleotide is repeated more times in individuals with HD than in normal individuals. In HD, mutant HTT is observed to affect neuronal function and structure leading to progressive neurodegeneration, however the exact disease mechanism is not well understood.
To study the function of HTT and the effects of mutant HTT protein in vivo, I will try to introduce trackable copies of HTT into mammalian cells, specifically neuronal cells. By overexpressing the HTT protein in varying amounts and with varying number of CAG repeats, we can try to elucidate the normal function of HTT in a non-disease context and examine how mutant HTT protein affects cell function and leads to neurodegeneration.
As seen in Jolene’s previous posts, it is can be difficult to express such a large protein in cells. Jolene attempted to express HTT in two brain cancer cell lines, but found limited success. I am using the same BacMAM virus system previously established at the SGC and attempting to apply it in the widely used neuroblast cell line SH-SY5Y. The protocols for the experiments described below and additional data can be found on Zenodo.
With limited examples of the use of BacMAM in SH-SY5Y in the literature, I decided to test a range of virus amounts and included a BacMAM GFP control which I could visualize under the microscope to see how effective it was in SH-SY5Y. In line with Jolene’s optimization experiments and a paper I found using BacMAM to overexpress wildtype and mutant LRRK2 implicated in Parkinson’s Disease (PMID: 22952710), I used up to 25% v/v of p3 (passage 3) BacMAM virus to transduce SH-SY5Y and included HEK293 for comparison.
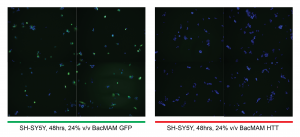
The LRRK2 article (PMID: 22952710) assayed cells for protein expression 48 hours post-transduction, so I thought I would get decent HTT expression within the same time-frame. I tracked GFP expression for 48 hours to get an overall idea of how effective the virus is in SH-SHY5. To detect HTT expression, I followed-up with FLAG-tag immunofluorescence (IF) in the SH-SY5Y (note: HEK293 don’t adhere very well and wash off easily so IF can’t be done with them). I find that GFP is very well expressed in both the HEK293 and SH-SY5Y cell lines after 48hrs, however, I did not detect any FLAG-tagged HTT in my IF (see RFP signal in figure below). This suggest that expression levels of the FLAG-tagged HTT from my BacMAM virus is likely too low to be detected using IF.
I decided to repeat the transduction in a larger 24-well format and harvest cells for a western to see if I could detect any HTT expression. I also changed the harvest point to 3 days post-transduction as Jolene successfully in detected overexpressed HTT in HEK293 with a longer 3-day post-transduction expression period. I observed some SH-SHY5 cells began dying 2 days post-transduction, with more cells dying and lifting off in higher v/v of BacMAM virus. After 3 days, I was only able to harvest the cells with 5% BacMAM virus for my western as most of the cells at higher v/v BacMAM virus died.
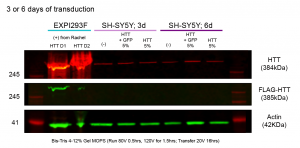
In my western, I detected FLAG-tagged HTT in the positive controls (D1 – 23 CAG repeats, D2 – 54 CAG repeats), but I did not detect any in both my SH-SY5Y grown for 3 days and 6 days post-transduction. It is possible that 5% v/v BacMAM virus is too low, but before I can move forward with higher v/v, I need to optimize conditions for the transduction in SH-SY5Y so that fewer cells die. The SH-SY5Y cells may be dying from either too much virus, the side effects of overexpressing HTT, or incompatibility with the Sf9 media the BacMAM virus is in. Moving forward, I will have to test these possibilities and optimize transduction conditions to maximize overexpression HTT while reducing cell death.
