Someone pointed out a funny quirk of mine the other day. I love things that come in threes. I never realized it before, but then I started thinking about it, and before you could say thirty-three, I was contemplating all the threes I like. Maybe its because the house I lived in growing up was 33 (a number to this day I have issues pronouncing, don’t ask), but I love things that come in threes, two always feels like too little, and four or more seems too much. When I go to McDonalds, I love the burger-fries-drinks combo; when I go to the movies I love the popcorn-candy-Drink combo, at restaurants its steak-fries-red wine. For breakfast I love tea-cornflakes-milk (I know reading this you think, man he is one exciting guy). My favorite dinosaur is the Velociraptor, with three claws per hand. Even when I write papers, I love titles with threes, if you want proof, check out my 3P paper. My favorite team has a world class top three. But its not just me, threes are everywhere, traffic lights come with three lights, movie series come in trilogies, hockey games have three periods, poutine has three ingredients (Can you name them?), the list goes on and on. So, three really is a magic number for me, which is why today’s protocol is based on three simple quality control steps for testing synuclein aggregates.
In my last entry, I spoke about making the synuclein protein, but today I will take it one step further and explain how once we aggregate the synuclein into fibrils, we do “3” simple checks to make sure its good to use in our experiments.
To make these aggregated fibril like structures, all we do is add synuclein into a tube and shake for 5-7 days. Once you are done, you have these long straw-like structures. And these are your fibrils. But they are way too long making them cumbersome to work with. So for our experiments, we like nice small, uniform fibrils, which is why we sonicate them. In a waterbath, we introduce a pulse-like vibration, that over 20 minutes breaks them into small pieces all of the same size. To come up with an analogy, if you ever had candy cane, smashing it into a hundred pieces to break it up, is like sonicating a synuclein fibril. This is pretty much sonication in a nutshell.
Once this is done, you now have three simple steps to make sure your fibrils are good.
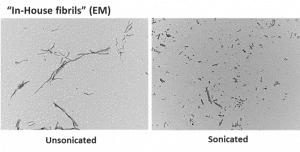
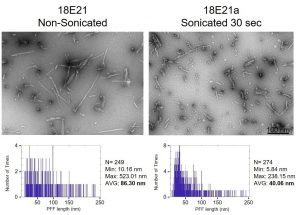
Step 1: Electron microscopy. Take some fibrils, both broken (sonicated) and not broken (unsonicated), and look at them under the electron microscope. With these images, you can measure the size, and determine whether they look good or not (Thanks to Dr. Esther Del Cild for the great EM images and quantification below). If something looks off, toss it out. This can mean inconsistent sizes, or fibrils are too long, or too small, or maybe you can’t even see them. Either way you have a problem with the batch you made.
Step 2: Dynamic Light scattering: This is a cuvette based system we have (explained in our protocol), that shines a light on our suspension of fibrils. Based on the way the light hits, we can measure the size and distribution of fibrils in our batches. If we see large variabilities, then we toss the batch, as we are looking for a batch as uniform in size as possible.
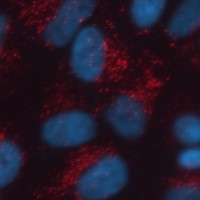
Step 3: Cellular uptake: if we are good in the first two steps, the last step is to check if the fibrils can get into cells. To do this, we add a fluorescent label (Alexa fluorescent dye) onto the fibrils, which provides us a quick and easy way to visualize the fibrils within the cells. Once we add the fibrils into the media the cells are grown in, they are rapidly taken up and after 24h we can image them in the cells. Below is an image of some fluorescent fibrils in Hela cells after only a few hours in the presence of cells. It was in the cell, as when cells are acid washed to remove any protein binding on its surface, the signal still remains intact, meaning the fibrils got into the cells.
Once we quantify the rate of uptake in Hela or other cells being used, and are happy with the size, shape and uptake of our fibrils, then we are ready to use them in our studies. And you can do, we make synuclein and synuclein PFFs for our projects, but also provide to other users, if your interested in some for your experiments, let me know and we can discuss how to help.
Well I hope you enjoying learning a little about my quirks and fascination with things that come in threes, next time we will learn about my favorite letter in my final blog of the summer. Next week is blog #10 and the end of my summer series, when I will cover one last protocol and fill you in on what’s coming next for me and my group.
